Lithium Bromide formula, also known as Lithium Monobromide formula or Bromolithium formula is discussed in this article. It is a lithium salt consisting of counterion bromide. The anhydrous salt forms cubic crystals that are similar to common salt. It is a combination of a lithium salt and bromide salt. The molecular or chemical formula of Lithium Bromide is LiBr.
Lithium Monobromide is synthesized by treating lithium carbonate (Li2CO3) with hydrobromic acid (HBr). The salt forms cubic crystals which are similar to sodium chloride. Hydrobromic acid and Lithium hydroxide precipitate lithium bromide (LiBr) in the presence of water.
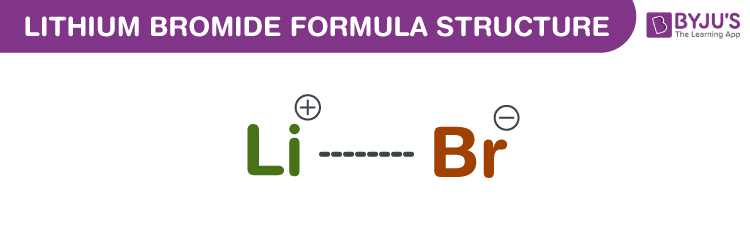
Lithium Bromide Formula Structure
Properties Of Lithium Bromide Formula
| Chemical formula | LiBr |
| Molecular weight | 86.845(3) g/mol |
| Density | 3.464 g/cm3 |
| Boiling point | 1,265 °C |
| Melting point | 552 °C |
Bromolithium is psychoactive and corrosive. When it is dissolved in water it generates heat due to the negative enthalpy of the solution.
To learn more about Lithium Bromide formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Lithium Monobromide for free.
Comments