Lithium hydroxide is an inorganic basic compound with a chemical formula LiOH. It is a white hygroscopic crystalline material that is soluble in water and slightly soluble in ethanol. It is largely used in organic synthesis to promote reaction due to its strong basicity. Lithium hydroxide is the only alkali hydroxide that does not show polymorphism, and its lattice possesses a tetragonal structure. In this short piece of article, let us discuss more about the lithium hydroxide formula, its properties and chemical structures along with its uses.
Properties of Lithium Hydroxide
| Lithium Hydroxide Properties | |
| Name | Lithium Hydroxide |
| Appearance | White solid |
| Molecular Formula | LiOH |
| Melting Point | 462 °C |
| Boiling Point | 924 °C |
| Density | 1.46 g/cm³ (anhydrous)
1.51 g/cm3 (monohydrate) |
| Molar Mass | 23.95 g/mol (anhydrous)
41.96 g/mol (monohydrate) |
Lithium Hydroxide Chemical Structure
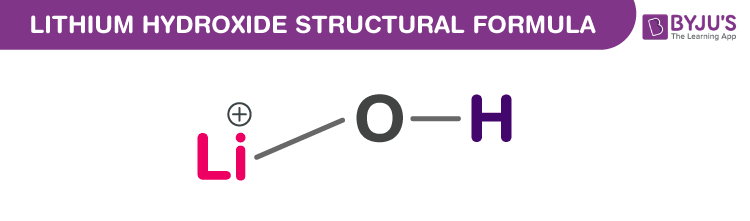
Lithium Hydroxide Uses
- Used in the production lithium greases
- Used in breathing gas purification system for submarines and spacecraft
- Used as a heat transfer medium and storage-battery electrolyte
To learn more about such chemistry topics register to BYJU’S now!
Comments