Magnesium Iodide formula, also known as Diiodomagnesium formula is discussed in this article. This chemical compound has various hydrates which are typical ionic halides and do not dissolve in water. The molecular or chemical formula of Magnesium Iodide is MgI2 (anhydrous).
Diiodomagnesium is a crystalline solid white in colour and has no smell. It can be synthesized by using magnesium oxide, magnesium carbonate, and magnesium hydroxide and treating them with hydroiodic acid. It is widely used to produce organic synthesis compounds.
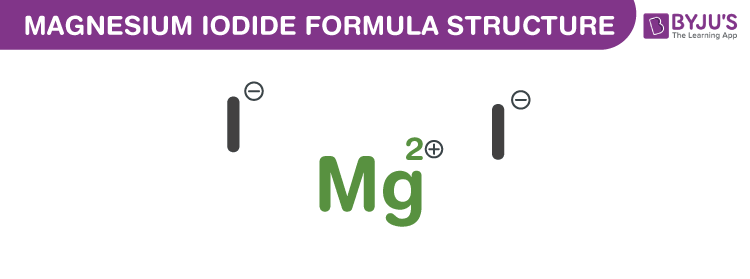
Magnesium Iodide Formula Structure
Properties Of Magnesium Iodide Formula
| Chemical formula | MgI2 (anhydrous) |
| Molecular weight | 278.1139 g/mol (anhydrous) |
| Density | 4.43 g/cm3 (anhydrous) |
| Appears as | crystalline solid white in colour |
| Melting point | 637 °C (anhydrous) |
It is a stable compound under a hydrogen atmosphere and in the presence of high heat. At normal temperature, it undergoes decomposition and turns brown in colour due to the elemental iodine release.
To learn more about Magnesium Iodide formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Diiodomagnesium for free.
Comments