Maltose is a disaccharide formed by two units of glucose with a chemical formula C12H22O11. Maltose is a component of a substance known as malt that is obtained from the process of allowing the grain to soften in water and germinate. In humans, maltose is broken down by maltase enzymes, producing two glucose molecules that can be further treated and can either be broken down to provide energy or can be stored as glycogen. In this short piece of article, learn about the maltose formula and its chemical structure in detail along with the uses of maltose.
Maltose Properties
| Properties of Maltose | |
| Name | Maltose |
| Also Known as | Malt Sugar, Malt Biose |
| Appearance | White powder or crystals |
| Chemical Formula | C12H22O11 |
| Melting Point | 102 °C to 1030C (monohydrate)
160 to 165 °C (Anhydrous) |
| Density | 1.54 g/cm³ |
| Molar Mass | 342.3 g/mol |
| Solubility in Water | Soluble |
Maltose Chemical Structure
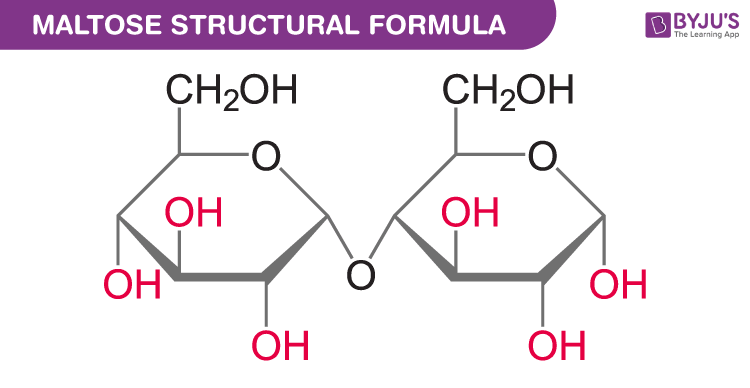
Maltose Uses
- Maltose is an excellent additive to packaged food including non-alcoholic beverages
- Adding maltose to food increases their shelf life
- Maltose can be used as a remedy for the dry mouth as it increases saliva production
- Maltose provides energy for the many bodily functions enabling us to perform our day-to-day tasks.
To learn more about such chemistry topics register to BYJU’S now!
Comments