NCERT Exemplar Solutions Class 10 Science Chapter 2 – Free PDF Download
The NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases and Salts is crafted by the subject experts at BYJU’S to assist students in understanding concepts in the chapter. Studying this Exemplar Solution will help you to gain knowledge of acids, bases and salts. You can access the NCERT Exemplar Class 10 Science Chapter 2 PDF below.
In this chapter, students will learn about a chemical reaction, writing and balancing chemical equations, types of chemical reactions, effects of oxidation reactions, and explanation of chemical reaction behind corrosion and rancidity. Acids, bases and salts are important as we use many of them in our daily lives. In order to handle them properly, it is vital to gain knowledge of acids, bases and salts. To guide you better on the topics, BYJU’S has brought NCERT Exemplars to you.
Download the PDF of NCERT Exemplar for Class 10 Science Chapter 2 – Acids, Bases and Salts
Access Answers of NCERT exemplar for Class 10 Science Chapter 2 – Acids, Bases and Salts
Multiple Choice Questions
1. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) The temperature of the solution increases
(ii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Soln:
The answer is (d) (i) and (iv)
Explanation:
When acid is mixed with a solution of base it results in a neutralization reaction. Neutralization is an exothermic reaction which results in the formation of salt.
2. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Soln:
The answer is (d) Hydrochloric acid
Explanation:
If the solution turns red litmus to blue colour then the solution should be basic in nature. Its effect can be neutralized by adding an acid hence (d) Hydrochloric acid is the answer.
3. During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Soln:
The answer is (c) absorb moisture from the gas
Explanation:
Calcium is a good dehydrating agent. It has the property to absorb moisture. Hence it is used as a desiccant to dry gases and Hydrocarbons in the industries.
4. Which of the following salts does not contain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Soln:
The answer is (b) Baking soda
Explanation
Baking sodas is white amorphous powder whereas other salts given in the question are crystalline in nature.
5. Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Soln:
The answer is (d) weak acid and strong base
Explanation:
Salt formed by weak acid and strong base form strong salt. Here, Sodium is a strong base and carbonate is a weak acid.
6. Calcium phosphate is present in tooth enamel. Its nature is
(a) basic
(b) acidic
(c) neutral
(d) amphoteric
Soln:
The answer is (a) basic
Explanation:
Phosphate ion present in calcium phosphate is a strong base and it forms a strong salt. Hence, calcium
phosphate is basic in nature.
7. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Soln:
The answer is (d) An antacid
Explanation:
Sample solution turn pH paper yellowish-orange which confirms the acidic nature of the sample. To make the colour to greenish-blue, we have to add an antacid.
8. Which of the following gives the correct increasing order of acidic strength
- Water < Acetic acid < Hydrochloric acid
- Water < Hydrochloric acid< Acetic acid
- Acetic acid< Water < Hydrochloric acid
- Hydrochloric acid< Water<Acetic acid
Soln:
The answer is a) Water < Acetic acid < Hydrochloric acid
Explanation:
Water is neutral in its pure form, Acetic acid is an organic acid which is weak in nature and Hydrochloric acid is a strong acid.
9. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
(a) Wash the hand with saline solution
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate
(c) After washing with plenty of water apply a solution of sodium hydroxide on the hand
(d) Neutralise the acid with a strong alkali
Soln:
The answer is (b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate
Explanation:
Washing affected hand with plenty of water will reduce the concentration of the acid. Remaining traces of the acid can be neutralized by applying a paste of Hydrogen carbonate which is basic in nature. Though NaOH is also a base but it is corrosive in nature hence it is not used to neutralize the acid.
10. Sodium hydrogen carbonate, when added to acetic acid, evolves a gas. Which of the following statements are true about the gas evolved?
(i) It turns lime water milky
(ii) It extinguishes a burning splinter
(iii) It dissolves in a solution of sodium hydroxide
(iv) It has a pungent odour
(a) (i) and (ii)
(b) (i), (ii) and (iii)
(c) (ii), (iii) and (iv)
(d) (i) and (iv)
Soln:
The answer is (a) (i) and (ii)
Explanation:
Reaction between Sodium hydrogen carbonate and acetic acid leads to the evolution of carbon-dioxide gas. CO2 turns the lime water milky and extinguish a burning splinter.
11. Common salt besides being used in the kitchen can also be used as the raw material for making:
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
(a) (i) and (ii)
(b) (i), (ii) and (iv)
(c) (i) and (iii)
(d) (i), (iii) and (iv)
Soln:
The answer is (a) (i) and (ii)
12. One of the constituents of baking powder is sodium hydrogen carbonate, the other constituent is
(a) hydrochloric acid
(b) tartaric acid
(c) acetic acid
(d) sulphuric acid
Soln:
The answer is (b) tartaric acid
Explanation:
A mild edible acid along with Sodium Hydrogen Carbonate is used to prepare baking powder. Here, acetic acid or citric acid can also be used in place of tartaric acid.
13. To protect tooth decay we are advised to brush our teeth regularly. The nature of the toothpaste commonly used is
(a) acidic
(b) neutral
(c) basic
(d) corrosive
Soln:
The answer is c) Basic
Explanation:
Teeth will be acidic in nature because of bacterial activity in the mouth. To neutralize the acid, toothpaste will be neutral in nature.
14. Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Soln:
The answer is (d) (ii) and (iv)
Explanation:
On a PH scale acids are those whose PH is below 7 lower is the PH stronger will be acid and similarly Bases are those whose PH is more than 7. Higher is the PH strong will be acid.
15. The pH of the gastric juices released during digestion is
(a) less than 7
(b) more than 7
(c) equal to 7
(d) equal to 0
Soln:
The answer is (a) less than 7
Explanation:
The PH is acidic to below 7 to ensure an easy breakdown of food particles. PH of stomach juices is usually 3.
16. Which of the following phenomena occur, when a small amount of acid is added to water?
(i) Ionisation
(ii) Neutralisation
(iii) Dilution
(iv) Salt formation
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Soln:
The answer is (b) (i) and (iii)
17. Which one of the following can be used as an acid-base indicator by a visually impaired student?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Soln:
The answer is (c) Vanilla essence
Explanation:
Vanilla essence can be used as an olfactory indicator hence it can be used as an acid-base indicator by visually impaired students.
18. Which of the following substance will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
Soln:
The answer is (d) Lime
Explanation:
Marble, Limestone and baking soda have carbonates which produces CO2 gas. Lime contains Hydroxide which will not produce CO2.
19. Which of the following is acidic?
(a) Lime juice
(b) Human blood
(c) Lime water
(d) Antacid
Soln:
The answer is (a) Lime juice
Explanation:
Lime juice has citric acid in it. Hence it is acidic in nature.
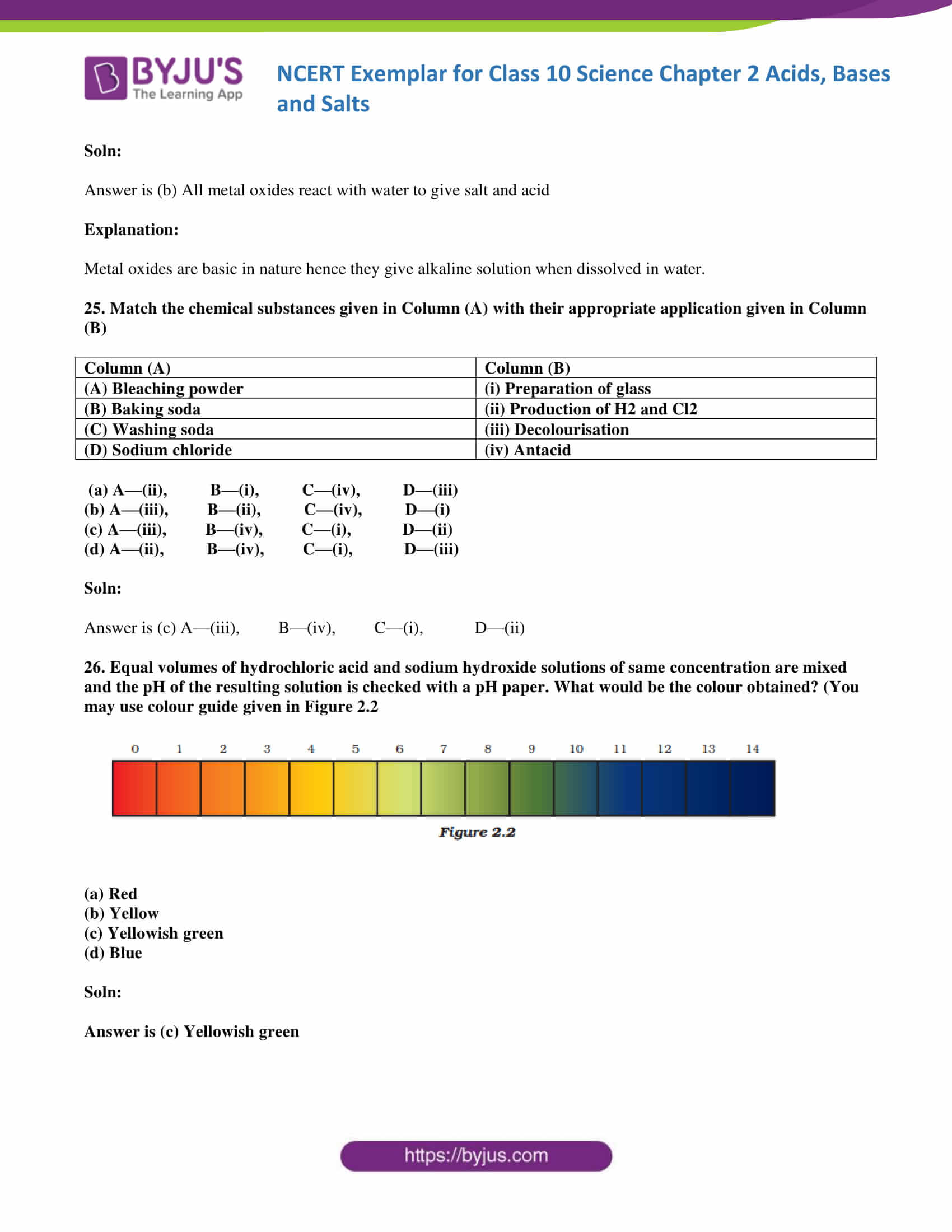
20. In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus
(Figure 2.1) was set up. Which among the following statement(s) is(are) correct?
- Bulb will not glow because the electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because the circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only

Soln:
The answer is (c) (ii) only
21. Which of the following is used for dissolution of gold?
(a) Hydrochloric acid
(b) Sulphuric acid
(c) Nitric acid
(d) Aqua regia
Solution:
The answer is (d) Aqua regia
Explanation:
Gold is a noble metal which will not react even with strong acids, hence aqua regia, which is a mixture of Nitric and Hydrochloric acid in the ratio 1:3 is used for dissolution of gold.
22. Which of the following is not a mineral acid?
(a) Hydrochloric acid
(b) Citric acid
(c) Sulphuric acid
(d) Nitric acid
Soln:
The answer is (b) Citric acid
Explanation:
Citric acid is the organic acid hence it is the answer
23. Which among the following is not a base?
(a) NaOH
(b) KOH
(c) NH4OH
(d) C2H5 OH
Soln:
The answer is (d) C2H5 OH
Explanation:
C2H5 OH is alcohol, not a base
24. Which of the following statements is not correct?
(a) All metal carbonates react with acid to give a salt, water and carbon dioxide
(b) All metal oxides react with water to give salt and acid
(c) Some metals react with acids to give salt and hydrogen
(d) Some non-metal oxides react with water to form an acid
Soln:
The answer is (b) All metal oxides react with water to give salt and acid
Explanation:
Metal oxides are basic in nature hence they give alkaline solution when dissolved in water.
25. Match the chemical substances given in Column (A) with their appropriate application given in Column (B)
| Column (A) | Column (B) |
| (A) Bleaching powder | (i) Preparation of glass |
| (B) Baking soda | (ii) Production of H2 and Cl2 |
| (C) Washing soda | (iii) Decolourisation |
| (D) Sodium chloride | (iv) Antacid |
(a) A—(ii), B—(i), C—(iv), D—(iii)
(b) A—(iii), B—(ii), C—(iv), D—(i)
(c) A—(iii), B—(iv), C—(i), D—(ii)
(d) A—(ii), B—(iv), C—(i), D—(iii)
Soln:
The answer is (c) A—(iii), B—(iv), C—(i), D—(ii)
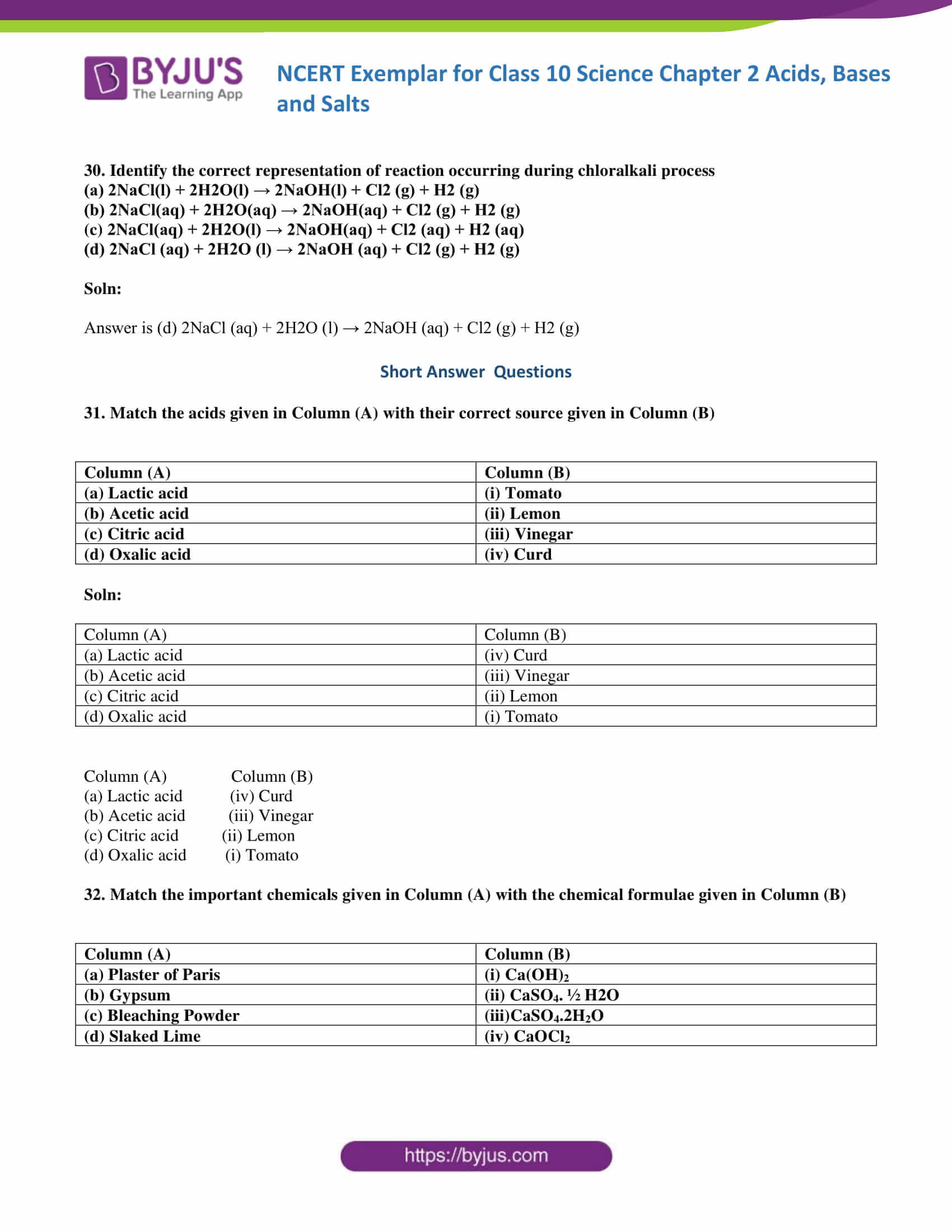
26. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained? (You may use colour guide given in Figure 2.2

(a) Red
(b) Yellow
(c) Yellowish green
(d) Blue
Soln:
The answer is (c) Yellowish green
Explanation:
Here neutralization takes place between HCL and NaOH solution hence PH will remain neutral which will be in
the yellowish-green zone in PH paper.
27. Which of the following is(are) true when HCl (g) is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution
(iii) It gives both hydrogen and hydroxyl ion in the solution
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
(a) (i) only
(b) (iii) only
(c) (ii) and (iv)
(d) (iii) and (iv)
Soln:
The answer is (c) (ii) and (iv)
Explanation:
HCL is a strong acid which ionizes completely in water to produce Hydrogen as well as Hydrogen and chlorine. Hydrogen produces combine with water molecules to give Hydronium ions.
28. Which of the following statements is true for acids?
(a) Bitter and change red litmus to blue
(b) Sour and change red litmus to blue
(c) Sour and change blue litmus to red
(d) Bitter and change blue litmus to red
Soln:
The answer is (c) Sour and change blue litmus to red
29. Which of the following are present in a dilute aqueous solution of hydrochloric acid?
(a) H3O+ + Cl–
(b) H3O+ + OH–
(c) Cl– + OH–
(d) unionised HCl
Soln:
The answer is (a) H3O+ + Cl–
Explanation:
Acid produces Hydrogen which will combine with a water molecule to produce Hydronium ion.
30. Identify the correct representation of reaction occurring during the chloralkali process
(a) 2NaCl(l) + 2H2O(l) → 2NaOH(l) + Cl2 (g) + H2 (g)
(b) 2NaCl(aq) + 2H2O(aq) → 2NaOH(aq) + Cl2 (g) + H2 (g)
(c) 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2 (aq) + H2 (aq)
(d) 2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Soln:
The answer is (d) 2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Short Answer Questions
31. Match the acids given in Column (A) with their correct source given in Column (B)
| Column (A) | Column (B) |
| (a) Lactic acid | (i) Tomato |
| (b) Acetic acid | (ii) Lemon |
| (c) Citric acid | (iii) Vinegar |
| (d) Oxalic acid | (iv) Curd |
Soln:
| Column (A) | Column (B) |
| (a) Lactic acid | (iv) Curd |
| (b) Acetic acid | (iii) Vinegar |
| (c) Citric acid | (ii) Lemon |
| (d) Oxalic acid | (i) Tomato |
Column (A) Column (B)
(a) Lactic acid (iv) Curd
(b) Acetic acid (iii) Vinegar
(c) Citric acid (ii) Lemon
(d) Oxalic acid (i) Tomato
32. Match the important chemicals given in Column (A) with the chemical formulae given in Column (B)
| Column (A) | Column (B) |
| (a) Plaster of Paris | (i) Ca(OH)2 |
| (b) Gypsum | (ii) CaSO4. ½ H2O |
| (c) Bleaching Powder | (iii)CaSO4.2H2O |
| (d) Slaked Lime | (iv) CaOCl2 |
Soln:
| Column (A) | Column (B) |
| (a) Plaster of Paris | (ii) CaSO4. ½ H2O |
| (b) Gypsum | (iii)CaSO4.2H2O |
| (c) Bleaching Powder | (iv) CaOCl2 |
| (d) Slaked Lime | (i) Ca(OH)2 |
33. What will be the action of the following substances on litmus paper? Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Soln:
Dry HCl gas- No effect
Moistened NH3 gas- Turns litmus paper to blue colour
Lemon juice- Turns litmus paper to red colour
Carbonated soft drink- Turns litmus paper to red colour
Curd- Turns litmus paper to red colour
Soap solution- Turns litmus paper to blue colour
34. Name the acid present in ant sting and give its chemical formula. Also, give the common method to get relief from the discomfort caused by the ant sting.
Soln:
Ant sting release methanoic acid. The chemical formula of methanoic acid is HCOOH. If we rub baking soda on the affected area, it can give relief from the discomfort caused by ant sting.
35. What happens when nitric acid is added to eggshell?
Soln:
Nitric acid dissolved eggshell, which is made up of Calcium carbonate. Calcium carbonate on reacting with nitric acid yield Calcium Nitrate and carbon-di-oxide gas.
36. A student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions, and litmus paper is not available in the laboratory. Since both the solutions are colourless, how will she distinguish between the two?
Soln:
Student can use Phenopthalein indicator to check the nature of the solution.
37. How would you distinguish between baking powder and washing soda by heating?
Soln:
When we heat baking powder CO2 is released which can be confirmed by passing evolved gas into lime water which will turn milky. This reaction will not happen when you heat washing soda.
38. Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water, and a gas C is evolved. The gas C, when passed through lime water, turns it milky. Identify A, B and C.
Soln:
Salt A is Baking soda (Sodium Hydrogen Carbonate) which will turn to Sodium carbonate( Salt B) on heating. Gas C turns lime water milky; hence it must be CO2.
39. In one of the industrial processes used for the manufacture of sodium hydroxide, a gas X is formed as a by-product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. Identify X and Y, giving the chemical equation of the reactions involved.
Soln:
X = Chlorine (Cl 2)
Y = Bleaching powder (CaOCl2)
Ca(OH)2 (s) + Cl2 (g) → CaOCl2 (s) + H2O — Calcium oxychloride (bleaching powder)
40. Fill in the missing data in the following table
| Salted Obtained from | |||
| Name of the Salt | Formula | Base | Acid |
| Ammonium chloride | NH4Cl | NH4OH | – |
| Copper sulphate | – | – | H2 SO4 |
| Sodium chloride | NaCl | NaOH | – |
| Magnesium nitrate | Mg (NO3 ) 2 | – | HNO3 |
| Potassium sulphate | K2 SO4 | – | – |
| Calcium nitrate | Ca(NO3 ) 2 | Ca(OH)2 | – |
Soln:
| Salted Obtained from | |||
| Name of the Salt | Formula | Base | Acid |
| Ammonium chloride | NH4Cl | NH4OH | HCl |
| Copper sulphate | CuSO4 | Cu(OH)2 | H2 SO4 |
| Sodium chloride | NaCl | NaOH | HCl |
| Magnesium nitrate | Mg (NO3 ) 2 | Mg(OH)2 | HNO3 |
| Potassium sulphate | K2 SO4 | KOH | H2SO4 |
| Calcium nitrate | Ca(NO3 ) 2 | Ca(OH)2 | HNO3 |
41. What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Soln:
Strong acids are those that get completely ionized and weak acids are those that get partially ionized.
Hydrochloric acid- Strong Acid
citric acid- Weak Acid
acetic acid- Weak Acid
nitric acid – Strong Acid
formic acid- Weak Acid
sulphuric acid- Strong Acid
42. When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved, which is utilised in the hydrogenation of oil. Name the gas evolved. Write the chemical equation of the reaction involved and also write a test to detect the gas formed.
Soln:
When Zinc reacts with Dilute acid solution, the following reaction takes place, and hydrogen gas is evolved
Zn + 2HCl→ ZnCl2 + H2
When Hydrogen gas is brought near a burning flame, it burns with a popping sound, which is the confirmation for the evolution of Hydrogen gas.
Long Answer Questions
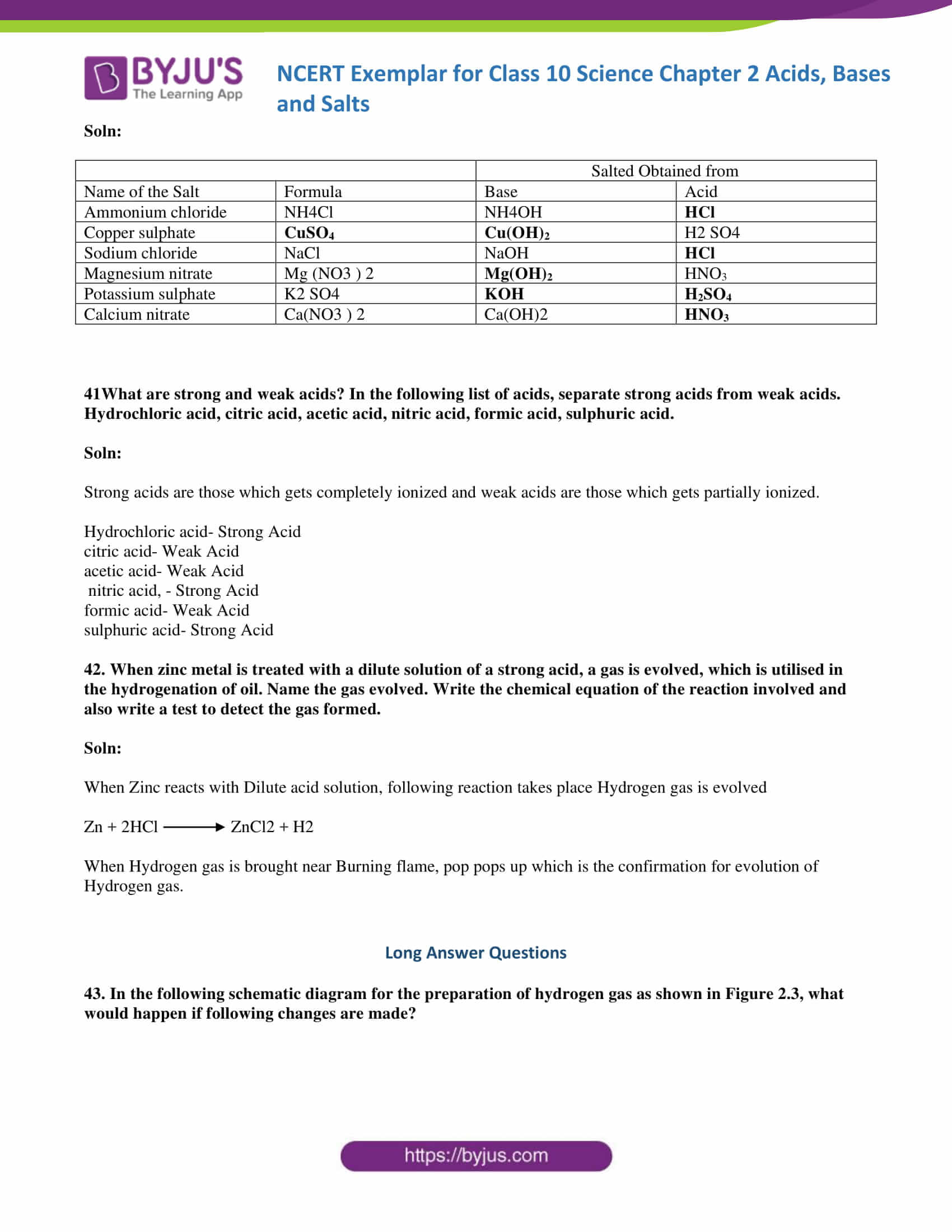
43. In the following schematic diagram for the preparation of hydrogen gas, as shown in Figure 2.3, what would happen if the following changes are made?

(a) In place of zinc granules, the same amount of zinc dust is taken in the test tube
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
(c) In place of zinc, copper turnings are taken
(d) Sodium hydroxide is taken in place of dilute sulphuric acid, and the tube is heated.
Soln:
- In place of Zinc granules if we use Zinc dust reaction speed increases
- Instead of dilute sulphuric acid if we use dilute hydrochloric acid ZINC Chloride is formed
Zn+ 2HCl→ ZnCl2+H2 - If we use copper in place of Zinc there will be no reaction as copper will not react with dilute acids
- Sodium Zincate is produced if we use NaOH solution in place of acidZn+2NaOH→ Na2ZnO2+ H2
44. For making a cake, baking powder is taken. If at home, your mother uses baking soda instead of baking powder in cake,
(a) how will it affect the taste of the cake and why?
(b) how can baking soda be converted into baking powder?
(c) what is the role of tartaric acid added to baking soda?
Soln:
a) If we use baking soda instead of baking powder taste of the cake will be bitter. Upon heating baking, soda sodium carbonate will be formed, which will make the cake taste bitter.
2NaHCO3 +Heat Na2Co3+CO2+H2O
b) Baking soda can be converted into baking powder by adding an edible weak acid like tartaric acid.
c) When tartaric acid is dissolved in water, it gives out Hydrogen ions. Hydrogen ions react with Sodium Carbonate to produce carbon dioxide, which will make the cake fluffy.
45. A metal carbonate X on reacting with acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at the anode during electrolysis of brine is passed on dry Y, and it gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
Soln:
X is Calcium. When calcium carbonate reacts with HCl, it gives out CO2 gas.
CaCO3 + HCl→ CaCl2+CO2+H2O
When CO2 is passed into lime water, it turns milky due to the formation of Calcium carbonate.
Hence, solution Y is lime water
When chlorine gas is passed on dry lime water, it gives bleaching powder which is used for disinfecting water.
46. A dry pellet of a common base B, when kept in the open absorbs moisture and turns sticky. The compound is also a by-product of the chloralkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Soln:
A compound which is a byproduct of the chloralkali process is NaOH. Hence compound B is NaOH.
When NaOH is treated with acidic oxide neutralization process occurs. For example, If NaOH is treated with carbon-di-oxide, it gives Sodium carbonate.
2NaOH+ CO2 →Na2CO3+ H2O
47. A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in the open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt, and why does it show such behaviour? Give the reaction involved.
Soln:
The sulphate salt should be calcium sulphate which is white and soft substance. Calcium sulphate is popularly known as plaster of Paris.
Plaster of Paris has half molecule of water of crystallization. When we leave plaster of Paris open for some time, it absorbs moisture to gain a number of molecules of crystallization. This newly formed compound is called Gypsum, which is hard to make moulds.
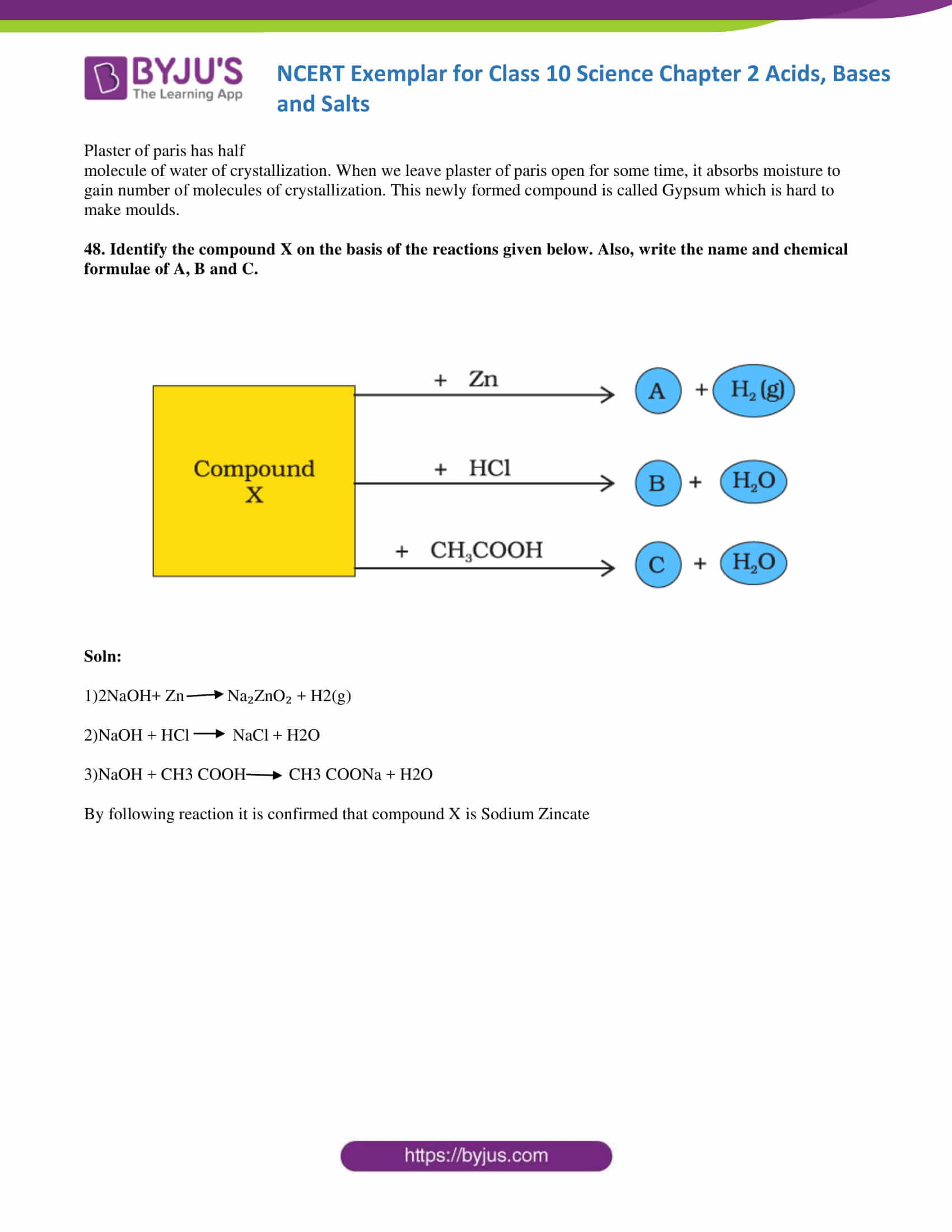
48. Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.

Soln:
1)2NaOH+ Zn→ Na₂ZnO₂ + H2(g)
2)NaOH + HCl →NaCl + H2O
3)NaOH + CH3 COOH→ CH3 COONa + H2O
By the following reaction, it is confirmed that compound X is Sodium hydroxide.
| Also Access |
| NCERT Solutions for Class 10 Science Chapter 2 |
| CBSE Notes for Class 10 Science Chapter 2 |
NCERT Exemplar Class 10 Science Acids, Bases and Salts
This exemplar has acid and base questions and answers, acids and bases multiple choice questions and answers, acid-base problems with answers, a list of natural indicators of acids and bases, and important questions from previous year question papers and sample papers. Students are also advised to solve previous years’ questions, as well as sample papers. Solving sample papers and previous years’ papers will also help them to know the exam pattern, as well as the marking scheme.
Important Topics of Chapter 2 Acid, Bases and Salts
- Understanding the chemical properties of bases and acids
- How do acids and bases react with metals?
- How do metal carbonates and metal hydrogen carbonates react with acids?
- How do acids and bases react with each other?
- The reaction of metallic oxides with acids
- The reaction of non-metallic oxide with base
- What do all acids and all bases have in common?
- What happens to an acid or base in a water solution?
- How strong are acid or base solutions?
- Importance of pH in everyday life
- More about salts
- Family of salts
- pH of salts
- Chemicals from common salt
Along with NCERT Exemplar Solutions, find notes, question papers, sample papers, NCERT Solutions and other study materials for all the subjects and classes. To get access to all study resources provided by us, register to BYJU’S or download BYJU’S – The Learning App.
Frequently Asked Questions on NCERT Exemplar Solutions for Class 10 Science Chapter 2
What is the pH of salt, as mentioned in Chapter 2 of NCERT Exemplar Solutions for Class 10 Science?
Why should I refer to NCERT Exemplar Solutions for Class 10 Science Chapter 2?
How can students score more marks using Chapter 2 of NCERT Exemplar Solutions for Class 10 Science?
Also Read











Comments