Table of Contents
- Overview
- Nitrogen Cycle
- Nitrogen Fixation Definition
- Mechanism of Biological Nitrogen Fixation – Nitrogen Fixation Process
- Nitrogen Fixation Reaction
- Nitrogen Fixation bacteria -Types and Examples
- Nitrogen Fixation by Rhizobium – Nodule Formation
- Nitrification
- Denitrification
Overview
Nitrogen is a vital component of all forms of life on earth. It forms the structural component of amino acids that constitute the animal enzymes, tissues and several hormones. Nitrogen accounts for approximately 78% of the atmospheric gasses yet needs to be fixed in order to be used by plants, why?
This is because the living forms are unable to use the free nitrogen gas available in the atmosphere. For nitrogen to be used for the synthesis of proteins, DNA and other significant compounds, it first needs to be converted into a form that can be used, a different chemical form. It occurs through nitrogen fixation.
Nitrogen Cycle
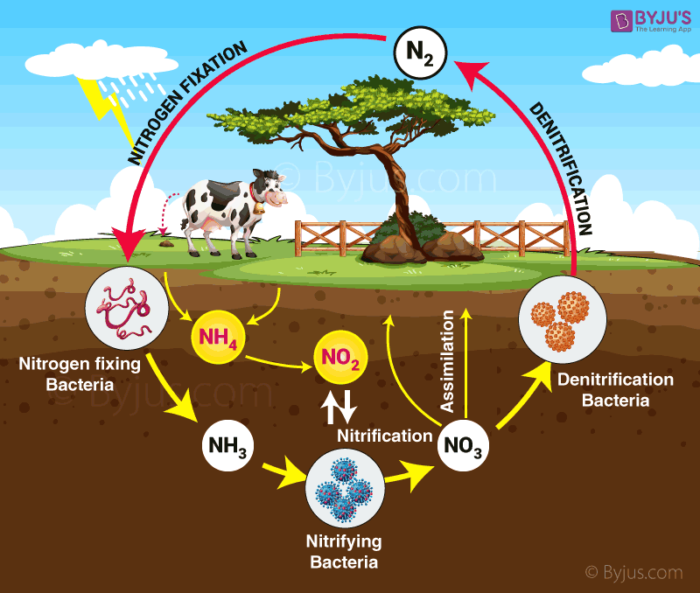
Nitrogen cycle is a biogeochemical process involving the conversion of free atmospheric nitrogen to a relatively accessible and usable form. It is a cyclic process. Nitrogen transforms into various forms in the cycle from the atmosphere to the ground.
Steps in nitrogen cycle –
- Nitrogen fixation
- Nitrification
- Nitrate assimilation
- Ammonification
- Denitrification and anammox
Nitrogen Fixation Definition
Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous compounds. The process can occur in the aquatic or the soil system.
Nitrogen present in the atmosphere is a comparatively non-reactive molecule, it chemically combines with other elements for the formation of more reactive compounds of nitrogen (nitrites, nitrates or ammonia). This process of nitrogen fixation is a vital microbially mediated phenomenon, converting dinitrogen into ammonia.
The process of conversion is brought about by some natural processes, and also carried out by some species of bacteria such as Azotobacter, Rhizobium etc.
Mechanism of Biological Nitrogen Fixation – Nitrogen Fixation Process
The process of biological nitrogen fixation can be indicated by the following equation, wherein 2 moles of ammonia are released from 1 mole of nitrogen gas at the expenditure of 16 moles of ATP accompanied by the provision of protons and electrons.
Nitrogen Fixation reaction
The process of biological fixation of nitrogen takes place when the atmospheric nitrogen is converted into ammonia by the enzyme nitrogenase.
The complete reaction for it is as follows –

The reaction is conducted by the prokaryotes in the presence of the nitrogenase enzyme, which in turn comprises two proteins – iron protein and a molybdenum-iron protein.
The phenomenon is combined with the hydrolysis of 16 equivalents of ATP and the formation of one equivalent of H2. The conversion of N2 into ammonia takes place at the iron-molybdenum cofactor (FeMoco).
The reaction takes place when the N2 binds with the nitrogenase enzyme complex. At first, the Fe protein is reduced by the electrons that are given by the ferredoxin. The reduced Fe then associates the ATP and reduces the molybdenum-iron protein that gives the electrons to N2 thus producing HN=NH. In the further cycles, eventually this is reduced to 2 NH3
How Do Plants Fix Nitrogen? – Nitrogen Fixation Plants
A few plants (as that of legumes) generate a symbiotic association with the nitrogen-fixing bacteria, referred to as rhizobia, known to infect the plant. Responding to the bacteria, it develops a structure referred to as the nodules on the roots (of the legumes).
Inside these root nodules, the symbiotic bacteria grow into organelles serving as nitrogen-fixing powerhouses, supplying nitrogen to the crop in return for carbon supplied by plants. This legume symbiosis is efficiently supplying and meeting all the nitrogen requirements of the plant.
Alongside the legume symbiosis, the soil bacteria capable of fixing nitrogen can also form associations with the cereal crops for their nitrogen needs. The phenomena can be optimized by the inoculation of the cereals with the nitrogen-fixing bacteria as the biofertilizers.
Nitrogen Fixation Bacteria – Examples
What are Nitrogen-fixing Bacteria?
The nitrogen-fixing bacteria essentially are prokaryotic microbes that can convert atmospheric nitrogen gas into nitrogenous compounds that can be used by plants.
These agents affect more than 90% of nitrogen fixation that occurs in nature, hence are vital in the nitrogen cycle.
Types of Nitrogen-fixing Bacteria
The nitrogen-fixing bacteria can broadly be classified into two main types –
- Symbiotic bacteria
- Non symbiotic bacteria
Before learning about the types of nitrogen-fixing bacteria, let us parallelly understand the symbiotic and non-symbiotic nitrogen fixation too.
Symbiotic Nitrogen Fixation
The symbiotic nitrogen fixation is the type of nitrogen fixation wherein the free atmospheric nitrogen is fixed by the symbiotic bacteria inhabiting the root nodules of the host plant. The bacteria and the host plant are in harmony as they maintain a mutually beneficial association. In the same soil, this very nitrogen that gets fixed can be used by the upcoming generation of legumes.
Symbiotic bacteria
These are mutualistic bacteria. For example – Rhizobium is associated with leguminous plants, some of the species of Azospirillum are linked with cereal grasses. The symbiotic nitrogen-fixing bacteria occupy the root hair of their host plant. It is in this very region they multiply, grow and trigger the formation of the characteristic root nodules. This results in the enlargement of the plant cells and the bacteria in such a close association.
The bacteria in the nodules convert the free atmospheric nitrogen to ammonia. This is used up by the host plant for growth and development, as the free atmospheric nitrogen could not be used up by the host plant. Now, in order to make sure the nodule formation is sufficiently growing and there is optimal growth of the legumes, it is a usual practice to inoculate the seeds with commercial cultures of the apt Rhizobium species. This is specifically carried out in the degraded soil or even in the soil that lacks the desired bacterium.
Non-symbiotic Nitrogen Fixation
The non-symbiotic type of nitrogen fixation involves the fixation of the free atmospheric nitrogen by the bacteria inhabiting the soil and that are free-living in nature. An example of non-symbiotic nitrogen-fixing bacteria is Clostridium pasteurianum, which also happens to be an anaerobic bacterium. Another example is Azotobacter chroococcum, which is an aerobic, free-living bacteria.
The process of non-symbiotic nitrogen fixation basically involves two steps –
- Ammonia-formation: it is formed by the reduction of atmospheric nitrogen. The conversion of ammonia into nitrites and nitrates can be done by nitrifying bacteria. Conversion of ammonia is brought about by the Nitrosospira, Nitrosococcus and Nitrosomonas
- Nitrification – conversion into nitrates is brought about by the Nitrospina, Nitrospir Nitrobacter
Non-symbiotic bacteria
These are free-living bacteria and include cyanobacteria, Nostoc, Anabaena and even the genera such as Clostridium, Beijerinckia and Azotobacter.
Importance of Nitrogen-Fixing Bacteria
Nitrogen is a vital component of nucleic acids and proteins essential for life on Earth. Though available in nature abundantly, it is not available in a readily accessible form to the plants (primary producers).
The nitrogen-fixing bacteria execute about 90% of the nitrogen fixation that occurs, and hence plays an instrumental role in the nitrogen cycle. It is courtesy of these bacteria that the legumes comprise the nitrogen required to synthesize proteins, in turn producing nutritious resources to be consumed by the consumers of the food chain. Further, the legumes and some of the cereal grasses are usually cultivated as green manure and also for crop rotation on fields.
Nitrogen Fixation by Rhizobium – Nodule Formation
The rhizobia and the leguminous plants mediate through the expression of the genes by the transmission of signals to activate the symbiotic genes in both the entities involved. Flavonoids, a type of phenolic, are released by the host plants into the rhizosphere. These flavonoids serve as the chemo-attractants for bacteria to the roots, and gradually rhizobial colonies fasten to the root hairs. The flavonoid signals activate the gene expression of the nodulation.
There are several nodulation genes in the rhizobial strains. The flavonoids on the surface of the rhizobial bacteria are identified by the expressed nodD protein. This nodD associates with a promoter DNA sequence, and hence activates the transcription of the nod genes of the operons.
A cluster of the nod genes encoding the enzymes generate the rhizobial nodulation signal. The roots identify the nod factor via its association to the surface protein receptor at the subapical root tip. This interpretation of the nod factor causes a growth in the root, generating distinct curling of the root hair trapping the rhizobia that sets up an infection of the root.
Now, the bacteria have access to the cell membrane of the plants. What follows is the invagination of the plasma membrane for the formation of the infection structure referred to as the infection thread. It is a tubular form stretching from the root hair tip all the way to the lower cells of the cortex.
Now, the rhizobia makes an entry into the infection thread, wherein it rapidly multiplies. Simultaneously, the cortex cells found underneath are rapidly proliferating for the formation of the nodule primordia. The infection thread extends into the cells of this nodule primordia.
Finally, the rhizobia are released into the nodule cell and enwrapped into a membrane that is obtained from the plasma membrane of the host cell. It is at this stage that the rhizobial bacteria become capable of fixing nitrogen.
Nitrification
The process of nitrification involves the conversion of ammonium to nitrate. The process is carried out by the nitrifying bacteria that obtain energy by oxidizing ammonium. This occurs during the use of carbon dioxide as its source of carbon for the synthesis of organic compounds. These entities are called chemoautotrophs. It obtains energy by chemical oxidation, and they are autotrophs as it is independent of organic matter.
The nitrifying bacteria are seen in atmospheres with moderate pH, however are not active in the soil having high acidity. Mostly, they occur as mixed-species communities, as a few of them are specialized in converting ammonium to nitrite. Some others are involved in the conversion of nitrite to nitrate.
Denitrification
The process of denitrification involves the conversion of nitrate to gaseous compounds by the action of microbes. Usually, the sequence includes the production of nitrite in the process. Different types of bacteria conduct this conversion when cultivated on the organic matter under anaerobic conditions. Due to the absence of oxygen for the usual aerobic respiration, it uses nitrate instead of oxygen as the end electron acceptor. This is referred to as anaerobic respiration.
When oxygen is absent, the reducible substance (nitrate here) serves a similar role and can be reduced to nitrous oxide, nitrite or N2. Consequently, The scenarios in which denitrifying entities are characterized are – the presence of oxidizable organic matter, presence of reducible nitrogen sources but the absence of oxygen. Some denitrifying bacteria are species of Alcaligenes, Pseudomonas etc.
You just read about nitrogen fixation, mechanism of biological nitrogen fixation, nitrifying bacteria, symbiotic and non-symbiotic fixation and nodule formation.
For related information and NEET preparation articles, visit NEET BYJU’S.
More here:
Comments