Nickel Nitrate is an attractive emerald green hygroscopic and odourless crystalline solid. It is a unique chemical compound that is non-combustible but has the ability to accelerate the burning of combustible materials. Nickel Nitrate is used in a nickel plating and is also used to get nickel catalysts for use in chemical production. Let us learn the chemical composition of Nickel Nitrate.
| Chemical formula | Ni(NO3)2 or N2NiO6 |
| Chemical names | Nickel(II) nitrate, Nickel dinitrate, Nickelous nitrate |
| Molecular weight | 182.701 g/mol (anhydrous)
290.79 g/mol (hexahydrate) |
| Density | 2.05 g/cm3 (hexahydrate) |
| Melting point | 56.7 °C (hexahydrate) |
| Boiling point | 136.7 °C (hexahydrate) |
| Solubility | Soluble in Ethanol |
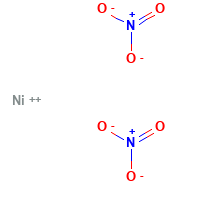
Nickel Nitrate Structural Formula
The structural formula for Nickel nitrate is as shown in the figure below. Here the nitrate ions are not bonded with nickel.
Nickel nitrate along with water is obtained when nickel oxide reacts with nitric acid
NiO + 2 HNO3 + 5 H2O → Ni(NO3)2.6H2O
Use Of Nickel Nitrate
- It is used as a precursor for nickel hydrazine nitrate which is an explosive.
Safety Measures
- Nickel nitrate is an oxidizing agent so need to handled carefully.
- It is carcinogenic and also causes skin allergy.
- It is a poison to aquatic organisms.
Stay tuned with BYJU’S to know more scientific information!!
Comments