Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. The changes could be physical such as melting, evaporation or could also be chemical such as burning, acid-base neutralisation etc.
- A calorimeter is what is used to measure the thermal changes of a body.
- Calorimetry is applied extensively in the fields of thermochemistry in calculating the enthalpy, stability, heat capacity etc.
What Is a Calorimeter?
A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. There is also a facility for stirring the contents of the vessel. This metallic vessel with a stirrer is kept in an insulating jacket to prevent heat loss to the environment. There is just one opening through which a thermometer can be inserted to measure the change in thermal properties inside. Let us discuss how exactly heat measurements are made. In the previous article, we discussed the specific heat capacity of substances.
Read More: Enthalpy
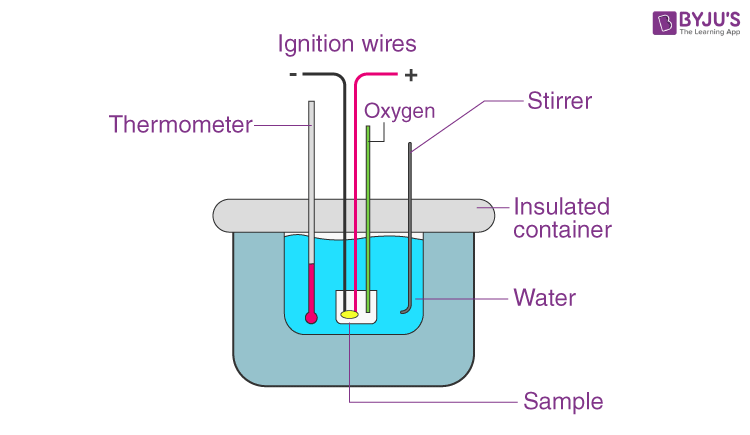
Such measurements can be made easily with this. Say in a calorimeter a fixed amount of fuel is burned. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Heat loss by the fuel is equal to the heat gained by the water. This is why it is important to insulate the calorimeter from the environment; to improve the accuracy of the experiment. This change in heat can be measured through the thermometer. Through such a measurement, we can find out both the heat capacity of water and also the energy stored inside a fuel.
Uses of Calorimetry
It is well known now that matter always obeys the principle of lowest energy i.e. given the option, the matter will exist in the lowest energy state possible. Despite this, matter can have a variety of energetic states. Uranium atoms, for example, are a powerhouse.
The energy of matter has a profound effect on its natural occurrence and its reactivity etc. If we can unravel the relationship between them, then we can predict the natural occurrence, reactivity and physical properties based on the energy measurements we make through calorimetry. Understanding the thermodynamic properties of a substance will inevitably yield answers to structure and other properties.
Read More: Principle of Calorimetry
Types of Calorimeter
Different types of calorimeters are given below:
- Adiabatic Calorimeters
- Reaction Calorimeters
- Bomb Calorimeters (Constant Volume Calorimeters)
- Constant Pressure Calorimeters
- Differential Scanning Calorimeter
If you wish to learn more Physics concepts with the help of interactive video lessons, download BYJU’S – The Learning App.

Comments