1
Question
Assertion :Benzene does not have true single and double bonds between its carbon atoms in the ring
Reason: It is composed of delocalized pi electrons in the ring giving rise to resonance structures
Assertion :Benzene does not have true single and double bonds between its carbon atoms in the ring
Reason: It is composed of delocalized pi electrons in the ring giving rise to resonance structures
Open in App
Solution
The correct option is A Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
Benzene does not have true single and double bonds between its carbon atoms in the ring, as the molecule is planar i.e all the carbon atoms show SP2 hybridization, and all the 6 pi electrons are delocalized in the ring exhibiting resonance.
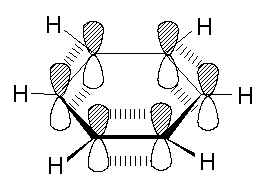
Benzene does not have true single and double bonds between its carbon atoms in the ring, as the molecule is planar i.e all the carbon atoms show SP2 hybridization, and all the 6 pi electrons are delocalized in the ring exhibiting resonance.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
