1
Question
From the reaction given below deduce the group of polonium in the periodic table (Pb belongs to group 14).
84Po210→82Pb206+2He4
From the reaction given below deduce the group of polonium in the periodic table (Pb belongs to group 14).
84Po210→82Pb206+2He4
Open in App
Solution
The correct option is D 16
Fajan, Russel and Soddy (1913) gave group displacement law which states that on the emission of an 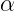 -particle the new element lies two columns left in the periodic table and mass number decreases by 4 units. Since, Pb belongs to group 14, Po must belong to group 16.
-particle the new element lies two columns left in the periodic table and mass number decreases by 4 units. Since, Pb belongs to group 14, Po must belong to group 16.
Fajan, Russel and Soddy (1913) gave group displacement law which states that on the emission of an
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
