1
Question
Explain structures of diborane and boric acid.
Explain structures of diborane and boric acid.
Open in App
Solution
Diborane
- Diborane is a chemical compound that consists of boron and hydrogen atoms and has a molecular formula .
- Structure of diborane:
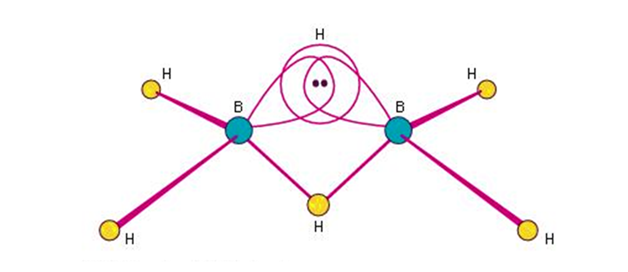
- The boron atoms has four hybrid orbitals and is considered to be hybridized.
- Three of the orbitals from these four hybrid orbitals have one electron each, and each one of them is an empty orbital.
- The two electrons of the hybrid orbitals in each of the boron atoms form 2 bonds with the 1s hydrogen atoms.
Boric acid
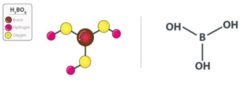
- Boric acid is a monobasic Lewis acid of boron with a chemical formula .
- It is an acid containing four atoms of oxygen, one atom of boron, and three atoms of Hydrogen.
- Structure of boric acid:
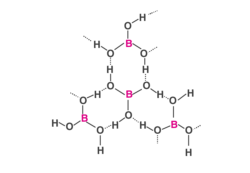
- Boric acid is formed in layers, the planar group is connected by H atoms to another unit.
- The H atoms form a covalent bond with the group and a Hydrogen bond with another unit.
- Structure:
Therefore, Diborane has the chemical formula whereas Boric acid has the chemical formula .
Diborane
- Diborane is a chemical compound that consists of boron and hydrogen atoms and has a molecular formula .
- Structure of diborane:

- The boron atoms has four hybrid orbitals and is considered to be hybridized.
- Three of the orbitals from these four hybrid orbitals have one electron each, and each one of them is an empty orbital.
- The two electrons of the hybrid orbitals in each of the boron atoms form 2 bonds with the 1s hydrogen atoms.
Boric acid
- Boric acid is a monobasic Lewis acid of boron with a chemical formula .
- It is an acid containing four atoms of oxygen, one atom of boron, and three atoms of Hydrogen.
- Structure of boric acid:

- Boric acid is formed in layers, the planar group is connected by H atoms to another unit.
- The H atoms form a covalent bond with the group and a Hydrogen bond with another unit.
- Structure:

Therefore, Diborane has the chemical formula whereas Boric acid has the chemical formula .
Suggest Corrections
21
