1
Question
What differs in the atomic structure of isotopes?
What differs in the atomic structure of isotopes?
Open in App
Solution
Isotopes:
- “Isotopes are atoms of the same element, which have different mass numbers”.
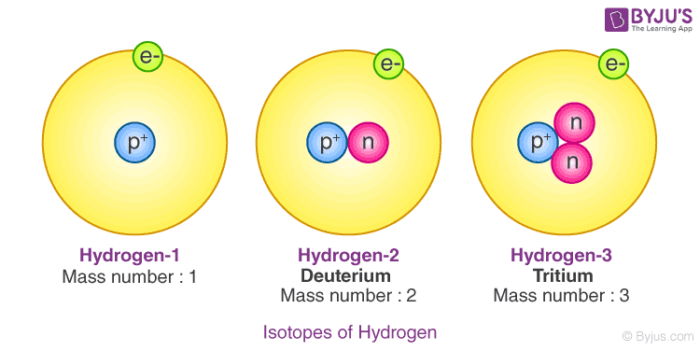
- Example: Consider the Hydrogen atom, which has three atomic species, namely Protium , Deuterium ,and Tritium .
- The difference in the number of neutrons in the nucleus makes the structure of isotopes different.
Isotopes of Hydrogen
- Isotopes of Hydrogen are shown below.
Isotopes:
- “Isotopes are atoms of the same element, which have different mass numbers”.
- Example: Consider the Hydrogen atom, which has three atomic species, namely Protium , Deuterium ,and Tritium .
- The difference in the number of neutrons in the nucleus makes the structure of isotopes different.
Isotopes of Hydrogen
- Isotopes of Hydrogen are shown below.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
