1
Question
What Is The Hybridization Of Ni In Ni Dmg 2 ?
What Is The Hybridization Of Ni In Ni Dmg 2 ?
Open in App
Solution
The hybridization of Ni in [Ni(DMG)2] is dsp2. Ni forms octahedral, square planar and tetrahedral complexes in +2 oxidation state.
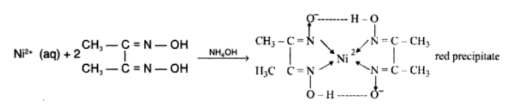
Ni2+ + 2DMG (dimethylglyoxime) → [Ni(DMG)2]↓ (bright red)
It acquires stability through chelation and intramolecular hydrogen bonding. In [Ni(DMG)2] the nickel is in the +2 oxidation state and to have a square planar geometry because of chelation the pairing of electrons takes place. So
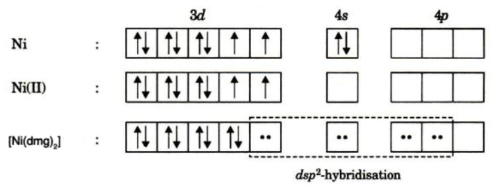
As all electrons are paired so complex is diamagnetic and nickel with coordination number four will have the structure as given below.
Reactions involved:
The hybridization of Ni in [Ni(DMG)2] is dsp2. Ni forms octahedral, square planar and tetrahedral complexes in +2 oxidation state.
Ni2+ + 2DMG (dimethylglyoxime) → [Ni(DMG)2]↓ (bright red)
It acquires stability through chelation and intramolecular hydrogen bonding. In [Ni(DMG)2] the nickel is in the +2 oxidation state and to have a square planar geometry because of chelation the pairing of electrons takes place. So

As all electrons are paired so complex is diamagnetic and nickel with coordination number four will have the structure as given below.
Reactions involved:

Suggest Corrections
15
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
