Students who want to look at extra questions and want to practice further will appreciate the Rajasthan Board Class 12 Chemistry Important Questions we have compiled here. After careful analysis of the previous year question papers, we have collected the types of questions covering those concepts that are more likely to appear in the board exams. They follow the question pattern and exam trends from the BSER Class 12 Previous Year Papers. If students are aiming to revise or even looking for a quick refresher of the concepts they have studied, then these important questions are the best source for them.
RBSE Class 12 Chemistry Important Questions
While the questions are given in this article, we also provide downloadable PDF format of these questions. Students can access them for future reference by clicking on the link given. Solving these RBSE Class 12 Chemistry Important Questions is the best way to ace the exams.
Download Rajasthan Board Class 12 Chemistry Important Questions PDF
1) Which type of semiconductor is formed when Arsenic is dropped with Germanium?
2) Write the electronic configuration of Chromium (Z=24).
3) Write the definition of Kohlrausch law of independent migration of ions.
4) Write the chemical equation of Cannizaro reaction.
5) What happens when a raw mango is placed in a concentrated salt solution.
6) Which series of compounds can be prepared by oxidation of primary alcohols?
7) Write IUPAC name of
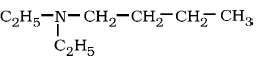 .
.
8) Explain that vulcanised rubber is an elastomer.
9) How will you convert sodium acetate to methane in one step?
10)What are thermosetting Polymers?
11) (a) Draw a neat and labelled diagram of a blast furnace.
(b) What is the role of Silica in the copper metallurgy
12) A solution of copper sulphate electrolyzed for 20 minutes with a current of 1.5 Ampere. Calculate the mass of copper deposited at the cathode. (F = 96500 C).
13) (a) When ammonia reacts with atmospheric oxygen in the presence of a catalyst.
(b) When sulphur acid reacts with sulphur trioxide
14) (a) Write any two differences between a soap and a detergent?
(b)In which work saccharine is used?
15) Classify the following into homoleptic and heteroleptic complexes?
(i) K4 [Fe (CN)6] (ii) [CO (NH3)5 (C03)]Cl
(iii) K2[ZN(OH)4] (iv) [Pt (NH3)2 Cl (NO2)]
16) Complete the following chemical reactions:
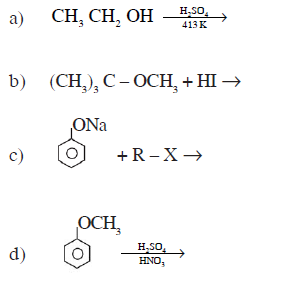
17) Explain the denaturation of protein with an example.
18) The osmotic pressure of a solution is 0.0821 atm at a temperature of 400k. Calculate the concentration of the solution in mol/litre.
19) What happens when the 1° and 2° alcohols are oxidised by anhydrous chromium trioxide (Cr O3)? Write chemical equations.
20) Give the order of the following reactions :
- Artificial radioactive decay.
(b) Decomposition of gaseous ammonia on a hot Pt-surface at high pressure.
(c) Hydrogenation of ethene.
21) Complete the following equations and identify A and B.
 .
.- Draw the resonating structures of Urea.
22) Read the paragraph and write the answers to the following questions:
Protein is very essential for the growth, development and maintenance of living systems. Proteins are natural polymers of α – amino acids. A definite sequence of amino acids forms a specific protein. Two or more than two amino acids bind to give peptide bond. Proteins are a polypeptide which loses its biological activity by physical or chemical changes.
(a)Explain essential and non-essential amino acid with example.
(b)Explain the denaturation of the protein.
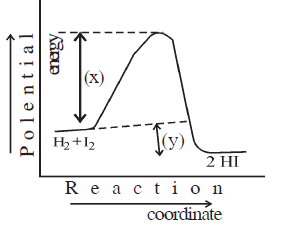
23) (a) Write suitable names of X and Y denoted in the below graph.
(b) Give a definition of only (A) denoted in the graph.
24) (a) Write the name of diseases due to deficiency of Vitamin “A” and Vitamin “B”.
(b) On the basis of “sugar” differentiate D.NA. and R.N.A.
25) Explain why Aryl Halides are less reactive towards nucleophilic substitution reaction.
26) (a) Due to the low concentration of oxygen in the blood, climber becomes weak and unable to think clear-
(i) Write the specific name of the above condition.
(ii)Explain the reason for such condition.
(b) 30gm of echanoic acid present in 100gm of water, determine molality of ethnoic acid in water.
27) (a) Write the values of axial angles for the hexagonal crystal system.
(b) Which type of semiconductor is obtained by doping boron with silicon? Explain.
28) (a) What are monosaccharides? Write the definition.
(b) (i) Name the product yields from the complete hydrolysis of DNA?
(ii)Write the structural formula of sugar moiety in DNA and RNA.
(c) Write the name of the method related to DNA to identify the dead body in an accident?
29) (a) What is anionic detergent? Write an example.
(b) Why are chemicals added to food? Write any two reasons.
(c) Write any one difference between broad-spectrum and narrow-spectrum antibiotics.
30) (a) Assuming that atoms are touching each other, calculate the efficiency of packing, in case of a crystal for simple cubic metal.
(b) On the basis of nature of iconic solids compare Frenkel defect and with Schottky defect.
31)
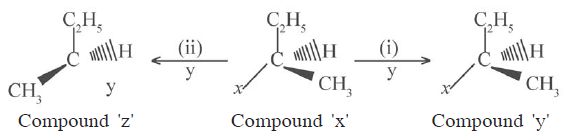
Write the names of the process to obtain compound (y) and compound (z) from compound (x) in the above unclophilin reaction (i) and (ii)
(b) Which type of nucleophilic substitution mechanism is involved to obtain compound (z) from compound (x). Write the name of the mechanism.
32) (a) Write the chemical equation to prepare sulphur dioxide from the industrial method?
(b) What happens when SO2 is passed through water?
(c)Write reaction showing reducing property of sulphur dioxide.
(d)Draw a labelled diagram showing ring structure of rhombic sulphur.
33) (a) What is enzyme catalysis? Write an example.
(b) Which type of emulsion is milk? Explain.
(c) Explain electrophoresis with a labelled diagram.
34) Explain the following with suitable example:
- Rosenmund Reaction
- Clemmensen Reduction
35) (a) Write a note on Wolff-Kishner Reduction.
(b) The boiling point of carboxylic acids are higher than aldehydes and ketones of comparable molecular masses. Why?
(c) Draw the dimer structure of ethanoic acid in the vapour state.
36) A) Write any two factors which affects the conductance of electrolysis.
B) Corrosion is an electrochemical phenomenon. Explain.
Comments