Chemistry is one of the significant subjects for Class 11 as it helps to create awareness about higher education avenues in the field of Chemistry, amongst students. The TN Board Class 11 Exam papers are designed to assess the level of understanding through multiple choice questions, numerical problems and so on.
TN Board Class 11 Chemistry Practicals
The final exams include marks assigned to theory question paper as well as practical. Here, for the convenience of the students we have compiled the experiments and activities from the Tamil Nadu Board Class 11 Chemistry Practicals. Students can find the experiments and observations in this downloadable link given here:
Download Tamil Nadu Board Class 11 Chemistry Practical PDFs
Students of Tamil Nadu Board can take a look at the practical experiments and observations given here to prepare well for the exams. The practical manual includes materials used as well as precautions and safety measures that students should observe to stay safe.
Tamil Nadu Board Class 11 Chemistry Practical Experiments
- Systematic analysis of a simple salt: Analysis of Anions
- Analysis with sodium carbonate extract
| List of Salts |
| 1. Lead Nitrate
2. Copper Sulphate 3. Copper Carbonate 4. Ferric Chloride 5. Zinc Sulphate 6. Zinc Sulphide 7. Aluminium Sulphate 8. Aluminium Nitrate 9. Calcium Carbonate 10. Barium Chloride 11. Ammonium Chloride 12. Ammonium Bromide 13. Magnesium Sulphate 14. Magnesium Carbonate 15. Magnesium Phosphate |
Find here the list of experiments, observations and inference:
Systematic analysis of a simple salt
Analysis of anions
Salt NO:
Date:
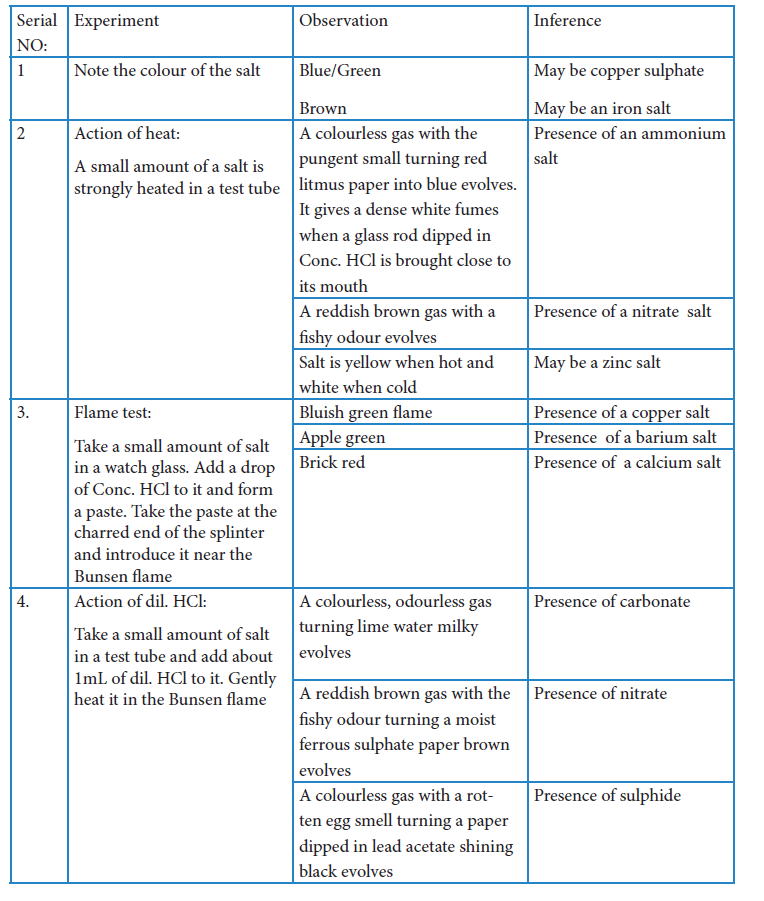

Analysis with sodium carbonate extract
Preparation of sodium carbonate extract:
Take 1g of the given salt and 3g of solid sodium carbonate in a 100mL beaker. Add 20g of
distilled water to it. Heat the beaker with its contents on a hot plate or Bunsen burner. After boiling the solution for few mins, filter it through a filter paper in a funnel and collect the filtrate in another beaker. The filtrate is called sodium carbonate extract.


Reasoning


Preparation of solution of the simple salt for the analysis of cations:
To a small amount of salt in a test tube add 2 to 3mL of water, shake it and gently heat it. If a
clear solution is obtained, directly use it for the analysis of cations. If the salt is insoluble, take a small amount of salt in another test tube, add 3mL of dil. HCl or dil. HNO3, shake it and gently heat it. If the salt dissolves, use the clear solution for the analysis of cations. This solution is called “original solution”.

The 3rd group metal ions form metal hydroxide ppt. The 4th group metal ions form metal sulphide ppt.


Reasoning:
Magnesium forms MgNH4PO4 ppt.
Maneson reagent is p-nitro azobenzene resorcinol. The blue ppt is due to precipitation of magneson by Mg (OH)3
Nessler’s reagent is prepared by slowly adding potassium iodide to mercury chloride. Initially a white ppt of HgI2 is obtained. The ppt dissolves in excess forming a clear solution. This clear solution is called the “Nessler’s reagent”. It is K2[HgI4]
The bown ppt is due to HgO.Hg(NH2)I. It is a basic mergury (ii) amido amine.
Analysis of group ppt:
Analysis of the 1st group ppt:
| Experiment | Observation | Inference |
| To the ppt add about
1mL of water and boil it |
The ppt dissolves | Presence of lead |
| Test for Lead:
i.) To one portion of the hot solution add about 1mL of K2CrO4 ii.) To another portion of the hot solution add about 1mL of KI. iii) To the yellow ppt add about 1mL of water, boil and cool. |
A yellow ppt is obtained
A yellow ppt is obtained The yellow ppt dissolves on boiling , and on cooling golden spangles appear |
Reasoning:
Lead forms PbCrO4 and PbI2 ppt – Recrystallisation of lead iodide crystals appeared as golden
yellow spangles.
Analysis of the 2nd group ppt:
To the ppt add about 1mL of dil HNO3 and boil it.The ppt dissolves. Cool it.
| i). To one portion of the solution add ammonium
hydroxide |
No ppt is obtained, but the
solution is blue |
Presence of copper |
| iii) Test for copper:To the blue coloured solution
add about 1mL each of acetic acid and potassium ferrocyanide |
A red brown ppt is obtained | Presence of copper |
Reasoning:
Prepare sodium stannite solution by mixing equal volume of about 1mL each of stannous chloride and sodium hydroxide.
With NH4OH copper forms soluble [Cu(NH3)4]2+ complex,
Copper forms a brown ppt of K2Cu[Fe(CN)6]
Analysis of the 3rd group ppt:
| To the ppt add a pinch of sodium peroxide and boil it | A red or brown ppt is obtained
A colourless solution is obtained |
Presence of iron
Presence of aluminium |
| i.) Test for iron:To one portion of the red ppt add about 1mL of diluted HCl and boil it and then add about 1mL of potassium ferrocyanide | A blue ppt is obtained | Presence of iron |
| ii.) To another portion of the ppt add about 1mL of dil. HNO3 boil it and then add about 1ml of KCNS | A blood red colouration is seen | Presence of iron |
| iii.) Test for aluminium:To the colourless solution add dil.HCl and shake it | A gelatinous white ppt is
obtained |
Presence of aluminium |
Reasoning:
Obtain sodium peroxide by mixing equal volume of about 1mL each of NaOH and H2O2
Iron forms a blue ppt (prussian blue) of Fe4[Fe(CN)6]3
Iron forms [Fe (CN)6]3- complex which is blood red coloured.
Aluminium forms a gelatinous white ppt of Al(OH)3
Analysis of the 4th group ppt:
| To the ppt add dil. HCl and boil it | The ppt dissolves | Presence of zinc |
| i.)Test for zinc To the solution add about 1.5mL of dil. NaOH and boil it | A clear solution is obtained | Presence of zinc |
Reasoning:
Zinc initially forms Zn (OH)2 ppt, and it dissolves in excess to form sodium zincate (Na2ZnO2)
Zinc forms white ppt of ZnS.
Analysis of the 5th group ppt:
| To the ppt add about 1mL of dil. Acetic acid and gently heat it. The ppt dissolves.
Divide the solution into two portions. i).To one portion add about 1mL of potassium chromate |
A yellow ppt is obtained. Filter the ppt using a funnel and filter paper, and transfer the residue to a watch glass. Add a drop of Conc. HCl. Take a portion of the paste at the charred end of a splinter and introduce near the Bunsen flame. A transient
green is imparted to the flame |
Presence of barium |
| ii). To an another portion add about 1mL of ammonium sulphate | A white ppt is obtained.
Filter the ppt using a filter paper and funnel. Transfer the residue to a watch glass. Add a drop of Conc. HCl. Take the residue at the charred end of the splinter and introduce near the Bunsen flame. A crimson red color is seen. If no ppt is obtained, to the solution add about 1mL of potassium ferrocyanide and shake it. A pale yellow ppt appears. |
Presence of calcium |
Reasoning:
Barium forms a yellow BaCrO4 ppt
The pale yellow ppt of calcium is due to Ca2[Fe(CN)6]
Students can also download the pdf given and print it out for future reference.
Stay tuned with BYJU’S and get further updates!
Comments