Carbon a chemical compound with atomic number 6. The best-known forms of carbon are diamond and graphite. Carbon is found abundantly next to oxygen in the human body and is the fourth plentifully found the chemical in the entire universe. It is the sixth element in the periodic table having the configuration of 1s22s22p2
Let us know the composition of carbon with various other details.
| Chemical formula | C |
| Atomic Number (Z) | 6 |
| Period and block | Period-2 and group-14 p-block |
| Electronic Configuration | [He] 2s2 2p2 |
| Electron per shell | 2,4 |
| Natural occurrence | Primordial |
| Structures | Exists in hexagonal and face-centred diamond-cubic |
| Oxidation State | -4,-3,-2,-1,0,+1,+2,+3,+4 |
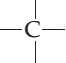
Carbon Structural Formula
The structural formula of carbon is as shown below. Carbon is a nonmetallic and tetravalent compound that can accommodate 4 electrons to form a covalent bond. It has the capacity to establish bonds easily with other atoms and with other carbon atoms too, carbon is competent of establishing multiple stable covalent bonds with suitable multivalent atoms.
Refer BYJU’S for informative videos and information!!
Comments