Aluminium sulfide or aluminium sulphide is a grey chemical compound with a chemical formula Al2S3. It readily hydrolyses in the air to form hydrogen sulfide. Aluminium sulfide is a greyish solid that posses a strong sulfide smell. It has six crystalline forms: α, β, γ and δ. It can be prepared through a thermite-like reaction between sulfur and aluminium powders. In this article, learn the aluminium sulfide formula and its chemical structure along with its properties and uses.
Aluminium Sulfide Properties
| Properties of Aluminium Sulfide | |
| Name | Aluminium Sulfide |
| Appearance | Gray solid with rotten egg smell |
| Chemical Formula | Al2S3 |
| Melting Point | 1,100 °C |
| Boiling Point | 1, 500 °C |
| Density | 2.02 g/cm3 |
| Molar Mass | 150.158 g/mol |
| Solubility in Water | Hydrolyses to release H2S |
Aluminium Sulfide Chemical Structure
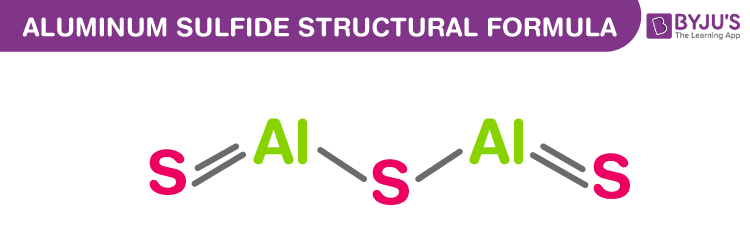
Aluminium Sulfide Uses
- Used in the preparation of hydrogen sulfide
- Used to produce chemicals used in the tanning and papermaking industry
- Used to produce organic compounds such as ethanethiol
To learn more about such chemistry topics register to BYJU’S now!
Comments