Ammonium bicarbonate formula, also named as Acid ammonium carbonate formula or Ammonium hydrogen carbonate formula is discussed in this article. It is an inorganic compound which is mildly basic. It consists of the ammonium cation and the bicarbonate anion. The molecular or chemical formula of Ammonium bicarbonate is NH4HCO3.
It appears as a crystalline solid white in colour. It has a strong smell of ammonia. It readily dissolves in water but insoluble in most various organic solvents. When Ammonium bicarbonate is dissolved in water it gives you a mildly alkaline solution.
Ammonium bicarbonate Formula Structure
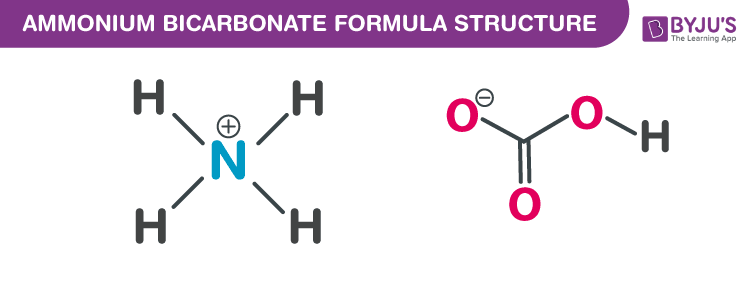
Properties Of Ammonium bicarbonate Formula
| Chemical formula | NH4HCO3 |
| Molecular weight | 79.056 g/mol |
| Density | 1.586 g/cm3 |
| Appearance | White crystalline solid |
| Melting point | 41.9 °C |
Use Of Ammonium bicarbonate
- It is widely used in some food processing applications, as an antacid, in cough syrups, as a fertilizer, like baking powder, pH buffer.
- It is also used to manufacture dyes, ceramics, production of pharmaceutical products, plastics, fire-retardants, etc.
To learn more about Ammonium bicarbonate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Acid ammonium carbonate for free.
Comments