Ammonium Bromide is odourless white crystals or granules which becomes yellow when exposed with air. Ammonium Bromide is the ammonium salt of hydrobromic acid. Let us learn the chemical composition and other details of Ammonium Bromide.
Properties Of Ammonium Bromide
| Chemical formula | NH4Br or BrH4N |
| Molecular weight | 97.943 g/mol |
| Density | 2.429 g/cm3 |
| Crystal structure | Isometric |
| Chemical Names | Ammonii bromidum, ammonium bromatum,
ammonium bromide ((NH4)Br) |
| Boiling point | 452 °C |
| Melting point | 235 °C |
Ammonium Bromide Structural Formula
Ammonium Bromide is an ammonium salt formed when ammonium ions react with bromide ions in a 1:1 ratio.
NH3 + HBr → NH4Br
The structural representation of Ammonium Bromide is shown as below.
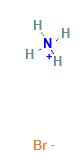
Use Of Ammonium Bromide
- It is used in photography.
- It is used in fireproofing wood.
- It is used as a corrosive inhibitor
Stay tuned with BYJU’S to know more about various chemical compounds.
Comments