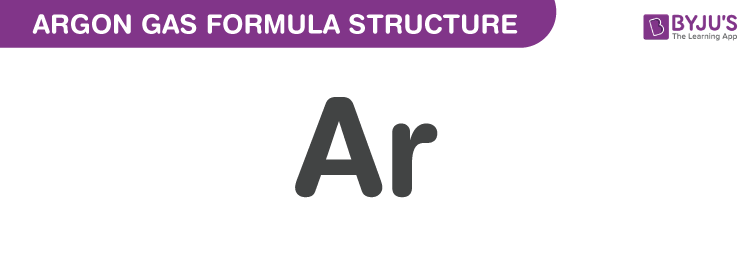
Argon Gas formula is discussed in this article. It is a noble gas with atomic number 18. It is monoatomic argon which is used in fluorescent tubes. The molecular formula of Argon Gas is Ar.
Argon is a colourless and odourless noncombustible gas. It is heavier than air. When exposed to prolonged heat or exposure to fire will cause violent rupturing. In its liquid form if it comes in contact with extremely cold water it can cause violent boiling. It plays an important role as a food packaging gas.
Argon Gas Formula Structure
Properties Of Argon Gas Formula
| Molecular formula | Ar |
| Molecular weight | 39.948 g/mol |
| Density | 1.784 g/L |
| Boiling point | −185.848 °C |
| Melting point | −189.34 °C |
Industrially it can be produced by fractional distillation of liquid air.
Uses Of Argon Gas
- It is widely used in welding as an inert shielding gas, to prevent the graphite from burning.
- It is used in fluorescent lighting, incandescent, and various other gas-discharge tubes.
To learn more about Argon Gas formula from the expert faculties at BYJU’S, register now!
Comments