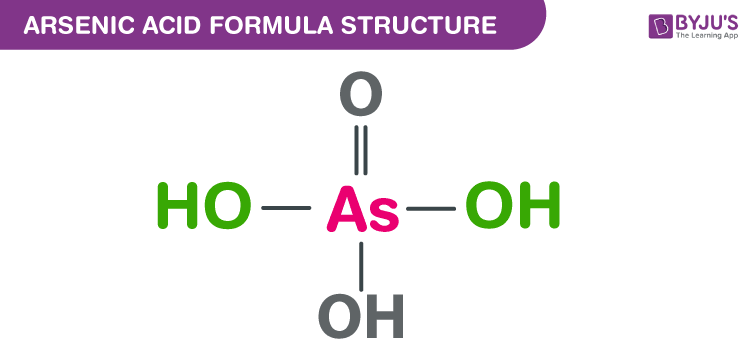
The arsenic acid formula, also named as Orthoarsenic acid formula or Arsoric acid formula is discussed in this article. It is an arsenic oxoacid which consists of three hydroxy groups and one oxo group. They are attached to the central arsenic atom. The molecular or chemical formula of Arsenic acid is H3AsO4.
In its aqueous form, it is a colourless solution. In its crystalline form, it is white and translucent. It is hygroscopic and non-combustible. It functions as an Escherichia coli metabolite. It is the conjugate acid of an arsenate ion and an arsenate(1-).
Arsenic acid Formula Structure
Properties Of Arsenic acid Formula
| Chemical formula | H3AsO4 |
| Molecular weight | 141.94 g/mol |
| Density | 2.5 g/cm3 |
| Boiling point | 120 °C |
| Melting point | 35.5 °C |
Use Of Arsenic Acid
- It is occasionally used in wood preservatives.
- It is used as a reagent in some dyes.
- It is used as a finishing agent in metal and glass.
Safety Measures Of Arsenic Acid
- It is corrosive and highly toxic when ingested.
- It is a human carcinogen and a threat to the environment and therefore immediate steps should be followed to limit the spread.
To learn more about Arsenic acid formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Orthoarsenic acid for free.
Comments