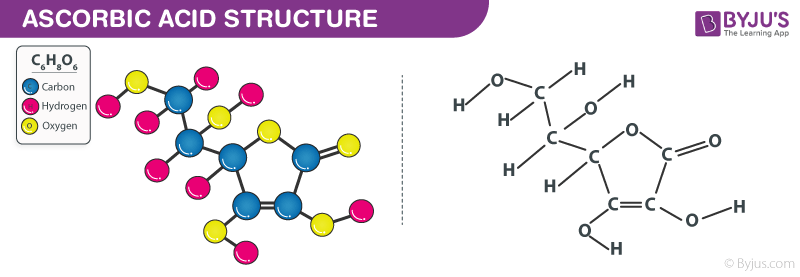
The ascorbic acid formula, also known as Hexuronic Acid formula or L-ascorbic acid formula is explained in this article. It is a six-carbon compound equivalent to glucose. A mild temperature, this compound reversibly changes from a polymer to a monomer. The chemical or molecular formula of Ascorbic Acid is C6H8O6.
In its solid form, it is white to pale yellow compound and easily dissolves in water. It is a mildly acidic solution. It acts as an antioxidant and is widely used in food additives. It is naturally found in many vegetables and citrus fruits. It is a mandatory nutrient in the human diet to maintain connective tissue. It functions as a coenzyme and reducing agent in metabolic pathways.
Ascorbic Acid Formula Structure
Properties Of Ascorbic Acid Formula
| Chemical formula of Ascorbic Acid | C6H8O6 |
| Molecular weight of Ascorbic Acid | 176.124 g/mol |
| Density of Ascorbic Acid | 1.65 g/cm3 |
| Appearance | Light yellow solid |
| Melting point of Ascorbic Acid | 190 to 192 °C |
Exposure to this compound will cause irritation in the eyes, skin, respiratory tract renal, and calcium oxalate calculi. Ingesting it will cause gastrointestinal distress. There have been cases of allergic reaction with eczema, urticaria, and asthma. On heating to decompose it emits irritating acrid smoke.
To learn more about Ascorbic Acid formula from the expert faculties at BYJU’S, register now!
Comments