Barium fluoride is a chemical compound composed of barium and fluorine and has a formula BaF2. It occurs in nature in the form of a mineral known as frankdicksonite. Here, in this short piece of article, learn more about barium fluoride formula, its chemical structure along with its properties and uses.
Barium Fluoride Properties
| Properties of Barium Fluoride | |
| Name | Barium Fluoride |
| Appearance | White cubic crystals |
| Molecular Formula | BaF2 |
| Melting Point | 1,368 °C |
| Boiling Point | 2,260 °C |
| Density | 4.89 g/cm³ |
| Molar Mass | 175.34 g/mol |
| Solubility in Water | Slightly soluble |
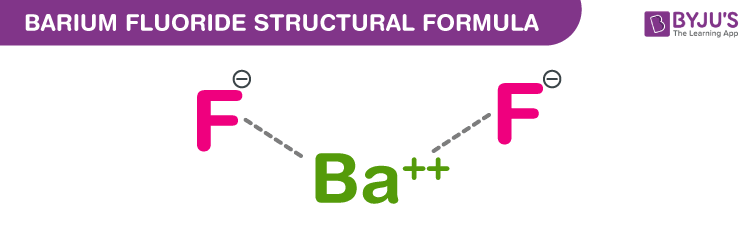
Barium Fluoride Chemical Structure
Barium Fluoride Uses
- Used to make optical components such as lens
- Used in the manufacture of carbon brushes for DC motor
- Used in the manufacture of glass
To learn more such chemistry topics register to BYJU’S now!
Comments