Oxidative Phosphorylation Definition
“Oxidative phosphorylation is the process of ATP formation, when electrons are transferred by electron carriers from NADH or FADH2 to oxygen”
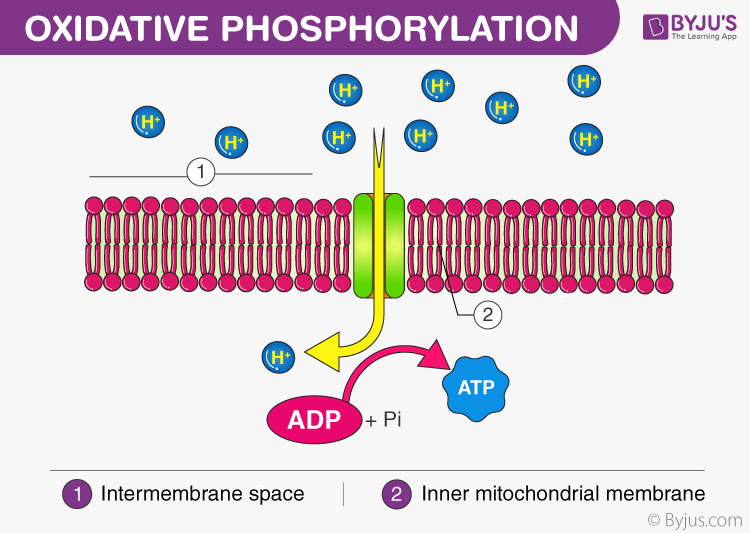
What is Oxidative Phosphorylation?
Oxidative phosphorylation is the final step in cellular respiration. It occurs in the mitochondria. It is linked to a process known as electron transport chain. The electron transport system is located in the inner mitochondrial membrane. The electrons are transferred from one member of the transport chain to another through a series of redox reactions.
Also Read: Amphibolic Pathway
Oxidative Phosphorylation Steps
The major steps of oxidative phosphorylation in mitochondria include:
Delivery of Electrons by NADH and FADH2
Reduced NADH and FADH2 transfer their electrons to molecules near the beginning of the transport chain. After transferring the electrons, they get oxidised to NAD+ and FAD and are utilised in other steps of cellular respiration.
Electron Transport and Proton Pumping
The electrons move from a higher energy level to a lower energy level, thereby releasing energy. Some of the energy is used to move the electrons from the matrix to the intermembrane space. Thus, an electrochemical gradient is established.
Splitting of Oxygen to form Water
The electrons are then transferred to the oxygen molecule which splits into half and uptakes H+ to form water.
ATP Synthesis
The H+ ions pass through an enzyme called ATP synthase while flowing back into the matrix. This controls the flow of protons to synthesize ATP.
Chemiosmosis
Oxidative phosphorylation uses the chemical reactions that release energy to drive a chemical reaction that requires energy. These 2 sets of reactions are coupled and interrelated.
The electrons that flow through electron transport chain is an exergonic process and the synthesis of ATP is an endergonic process. These two processes are ingrained within a membrane. As a result, energy will be transmitted from the electron transport chain to ATP synthase by the movement of proteins. This process is termed as chemiosmosis.
Endergonic Process is a chemical reaction in which energy is absorbed. There will be a change in free energy and it is always positive. Exergonic Process is a chemical reaction in which there will be a positive flow of energy from the system to the surrounding environment. Chemical reactions are also considered exergonic when they are spontaneous.
Electron Transport Chain
Most of the biochemical catabolic processes like the citric acid cycle, glycolysis, beta-oxidation, etc. produce the coenzyme NADH. It consists of electrons having high transfer potential.
These reactions release a huge amount of energy on oxidation. These reactions are also known to be the uncontrollable reactions since the energy within the cells is not released at once.
The electrons are separated from the NADH and then passed to the oxygen with a series of enzymes releasing a small amount of energy. All these series of enzymes having complexes is known as electron transport chain.
This chain can be seen in the inner layer or membrane of mitochondria. The salts of succinic acid are also oxidized by this electron chain transport system.
In the case of eukaryotes, the enzymes make use of energy that has been released in the electron transport system from the oxidation of NADH that pumps protons across the inner membrane of the mitochondria. This results in the generation of the electrochemical gradient across the membrane. This can be considered as one of the best examples to understand the concept of oxidative phosphorylation.
Also Read: TCA Cycle
Stay tuned with BYJU’S to learn more about what is oxidative phosphorylation, its definition, steps and other related topics.

Comments