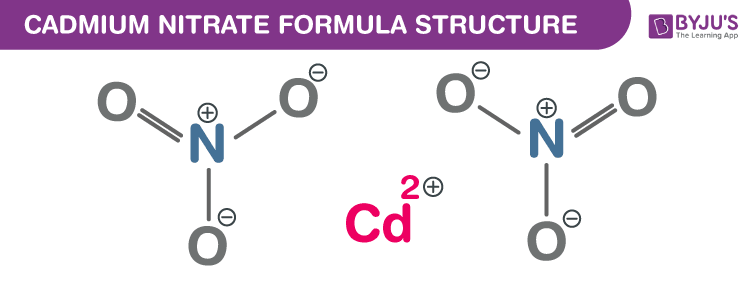
Cadmium nitrate formula, also named as Cadmium (II) nitrate formula or Cadmium dinitrate formula is discussed in this article. An inorganic crystalline compound which is colourless. It produces toxic cadmium oxide fumes on heating. The molecular or chemical formula of Cadmium nitrate is Cd(NO3)2.
When exposed to this compound it causes severe irritation to the skin, respiratory tract, and eyes. Also, damages the lungs leading to chest pain, shortness of breath. It can also cause damage to kidneys resulting in decreased renal function. It is a known carcinogen with an elevated risk to develop lung cancer.
Cadmium nitrate Formula Structure
Properties Of Cadmium Nitrate Formula
| Chemical formula | Cd(NO3)2 |
| Molecular weight | 236,42 g/mol |
| Density | 3.6 g/cm3 (anhydrous)
2.45 g/cm3 (tetrahydrate) |
| Boiling point | 132 °C (tetrahydrate) |
| Melting point | 360 °C (anhydrous)
59.5 °C (tetrahydrate) |
Use Of Cadmium Nitrate
- It is widely used in the production of cadmium hydroxide.
- It is used in to colour porcelain and glass.
- Used in nuclear reactors.
- It is widely used in photography.
To learn more about Cadmium nitrate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Cadmium (II) nitrate for free.
Comments