Calcium Bromide formula, also known as Calcium Dibromide formula or Calcium Bromatum formula is explained in this article. Calcium Bromide is formed by one calcium atom and two bromine atoms. The molecular or chemical formula of Calcium Bromide is CaBr2.
In its anhydrous form, it anhydrous is hygroscopic colourless crystals sharp saline taste. It is soluble in water and alcohol. It is produced by reacting calcium carbonate (CaCO3) and calcium oxide with hydrobromic acid (HBr). Or it is made by reacting calcium metal with elemental bromine. It is widely used as a dense aqueous solution for drilling fluids. Also, it has a wide application in as neuroses medication, food preservatives, freezing mixtures, fire retardants, and in photography.
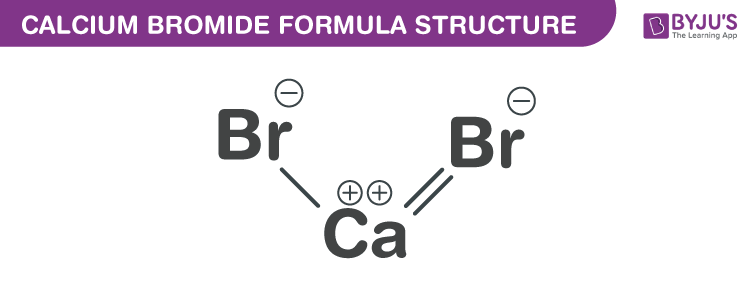
Calcium Bromide Formula Structure
Properties Of Calcium Bromide Formula
| Chemical formula | CaBr2 |
| Molecular weight | 199.89 g/mol (anhydrous)
235.98 g/mol (dihydrate) |
| Density | 3.353 g/cm3 |
| Boiling point | 1,815 °C (anhydrous)
810 °C (dihydrate) |
| Melting point | 730 °C |
Calcium Dibromide non-damaging to any formation, and is chemically as well as thermally stable. Other solutions containing chlorides and bromides can be blended with this compound.
To learn more about Calcium Bromide formula from the expert faculties at BYJU’S, register now!
Comments