What is Calcium Carbonate?
Calcium carbonate is an odourless chemical compound. It is a water-insoluble source of calcium. It is mainly found in rocks and is the carbonic salt of calcium.
Some of the pure calcium carbonate minerals are Calcite, Vaterite, Aragonite. Biological sources of calcium carbonate are Snail shells, Eggshells, Oyster shells etc. Mostly used as an antacid or calcium supplement. PH value is about 9.91. Its common name is limestone.
Table of Contents
- Preparation of Calcium carbonate
- Properties of Calcium carbonate
- Calcium carbonate structure
- Calcium Carbonate uses
- Frequently Asked Questions – FAQs
Preparation of Calcium Carbonate CaCO3
Calcium carbonate is primarily mined and processed from diverse natural mineral sources. It can also be made chemically by combining quicklime (calcium oxide, CaO) with water to produce calcium hydroxide (Ca(OH)2), which is then treated with carbon dioxide to form the calcium carbonate salt.
CaO + H2O → Ca(OH)2
Ca(OH)2 + CO2 → CaCO3 + H2O
It can be prepared on a large scale by passing carbon dioxide gas through calcium hydroxide (otherwise called slaked lime). However, if there is an excess passing of carbon dioxide, it results in the formation of soluble calcium hydrogen-carbonate.
Properties of Calcium Carbonate CaCO3
| Sodium bicarbonate Chemical formula | CaCO3 |
| Molecular Weight/ Molar Mass | 100.0869 g/mol |
| Density | 2.71 g/cm³ |
| Boiling Point | Decomposes at 899 °C |
| Melting Point | 825 °C |
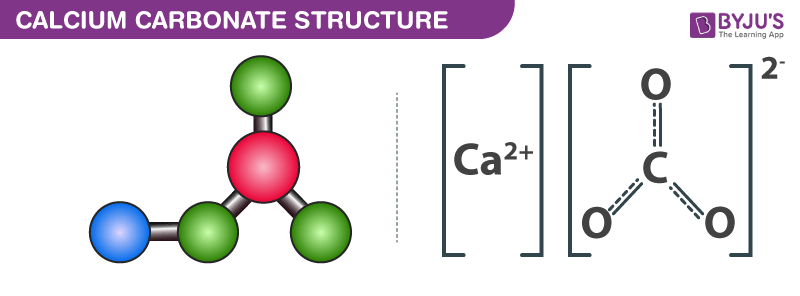
Calcium Carbonate Structure CaCO3
Calcium Carbonate uses
- It is used as a dietary supplement when the quantity of calcium in the diet is less
- It is used as an antacid to relieve stomach upset, heartburn
- It is used in to purify iron from iron ore
- It is used in the refining of sugar
- It is used in agriculture to neutralize acidic soil
- It is used as scrubbing agent in household cleaning powders
- It is used in the construction of buildings and roads
Frequently Asked Questions – FAQs
What are the uses of calcium carbonate?
Calcium carbonate is a dietary supplement that is used when the calcium contained in the diet is not adequate. For healthy bones, muscles, nervous system and heart, the body requires calcium. Often, calcium carbonate is used as an antacid to relieve heartburn, acid indigestion and stomach discomfort.
Is calcium carbonate acidic or basic?
Calcium carbonate is the salt of a strong base (due to the presence of the calcium ion) and a weak acid (due to the presence of the carbonate ion, usually derived from carbonic acid). Therefore, this salt is mildly basic in nature.
How is calcium carbonate prepared?
Mining or quarrying activities are responsible for the production of a vast majority of calcium carbonate used in industry. It is possible to obtain pure calcium carbonate (e.g. for food or medicinal use) from a pure quarry source like marble. Calcium carbonate can also be prepared from calcium oxide.
What happens if you have too much calcium carbonate?
Calcium carbonate is not very poisonous. Recovery is quite likely. But, long-term overuse is more serious than a single overdose, because it can cause kidney stones and more serious damage to kidney function. High calcium levels can also cause serious heart rhythm disturbances.
What are the benefits of calcium tablets?
Calcium is key to growing new bone and keeping the bone you have strong. Calcium supplements are standard for treating and preventing osteoporosis — weak and easily broken bones — and its precursor, osteopenia. Calcium has many other uses. It’s an ingredient in many antacids.


Comments