What is Acetonitrile?
Acetonitrile is a nitrile which is a hydrogen cyanide where the hydrogen (H) is replaced by a methyl group (-CH3).
Acetonitrile is a volatile organic compound. It is also called Cyanomethane or Methanecarbonitrile. The chemical formula of Acetonitrile is C2H3N.
Table of Contents
- Structure of Acetonitrile
- Properties of Acetonitrile
- Chemical Properties of Acetonitrile
- Uses of Acetonitrile
- Health Hazards
- Frequently Asked Questions – FAQs
Cyanomethane is a limpid liquid which has no colour which has an aromatic odour. It has a flash point value of 42°F and is less dense when compared to water. Its vapours are denser when compared to air. Methanecarbonitrile has a burning sweetish taste and is readily soluble in water. It functions as a polar aprotic solvent and is an aliphatic nitrile.
Structure of Acetonitrile (C2H3N)
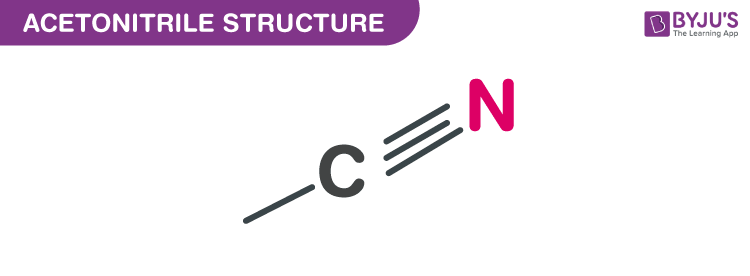
Structure of Acetonitrile – (C2H3N)
Properties of Acetonitrile – C2H3N
| Acetonitrile | C2H3N |
| Molecular Weight of Acetonitrile | 41.053 g/mol |
| Density of Acetonitrile | 0.786 g/cm3 |
| Melting Point of Acetonitrile | −46 to −44 °C |
| Boiling point of Acetonitrile | 81.3 to 82.1 °C |
Chemical Properties of Acetonitrile (C2H3N)
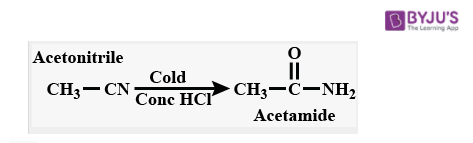
1. Reaction with Concentrated HCl
Upon partial hydrolysis of acetonitrile in cold concentrated HCl, acetamide product is formed. On complete hydrolysis, it forms a carboxylic acid.
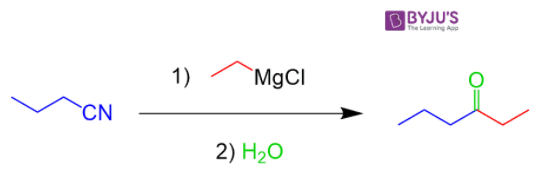
2. Reaction with phenyl magnesium bromide
Nitriles react with Grignard reagent to form ketones. Nitriles, RC≡N, react with Grignard reagents or organolithium reagents to give ketones. The strongly nucleophilic organometallic reagents add to the C≡N bond in a similar fashion to that seen for aldehydes and ketones.
Uses of Acetonitrile (C2H3N)
- Acetonitrile is used as a solvent in the extraction process of hydrocarbons.
- It is used to separate fatty acids from vegetable oil.
- Used in making perfumes.
- It is widely used in the production of synthetic pharmaceuticals.
- It is used in the manufacturing of rubber.
- It is used in refining as well as extraction of copper.
- Used as a solvent in electrochemical cells.
Preparation of Acetonitrile
It is obtained as a byproduct by manufacturing acrylonitrile. It can also be synthesized by hydrogenation of mixtures of ammonia and carbon monoxide or dehydration of acetamide.
Health Hazards
This compound is toxic through skin absorption. It is highly flammable and gives off toxic gases or fumes in a fire. Cyanomethane air or vapour mixtures are explosive. It can explode when it comes in contact with any strong oxidizing agent. It can burst due to a rise in pressure when heated. Severe exposures can cause skin eruptions, delirium, paralysis, irritation, confusion, and convulsions.
Frequently Asked Questions – FAQs
What is acetonitrile used for?
Acetonitrile is used for the manufacture of pharmaceuticals, perfumes, rubber goods, pesticides, batteries and acrylic nail removers. This is also used for extracting fatty acids from vegetable and animal oils.
Why is acetonitrile a good solvent?
Acetonitrile is a strongly polar solvent in its solvability, comparable to alcohols. Like alcohols, it is not a donor of hydrogen bonds but is a strong acceptor of hydrogen bonds. Acetonitrile nitrogen is quite weakly basic but in this respect, it can be very nucleophilic, quite similar to pyridine.
Is acetonitrile more polar than methanol?
Methanol is a polar-protic solvent, while acetonitrile is a polar aprotic solvent and has a better moment in the dipole.
Is acetonitrile a strong base?
In the presence of a strong aqueous base, such as NaOH or KOH, acetonitrile, a common solvent in organic synthesis, can be hydrolyzed which can spread into a runaway reaction.
Are acetone and acetonitrile the same?
All acetonitrile and acetone are organic compounds, with different chemical structures and physical and chemical properties. Acetonitrile is a nitrile compound, while acetone is a ketone.

Comments