Acetylene formula or ethyne formula is given here along with its structural and chemical formula. The chemical and structural formula for acetylene os given and explained in the following points. It should be noted that acetylene is a hydrocarbon and is the simplest alkyne. It has two carbon atoms which are bounded by a triple bond which makes acetylene as an unsaturated compound.
Structural Formula for Acetylene
Acetylene has two triple bonded carbon atoms and two hydrogen atoms attached to each one them. Check the structure of acetylene to understand this compound better. The acetylene structural formula is given below.
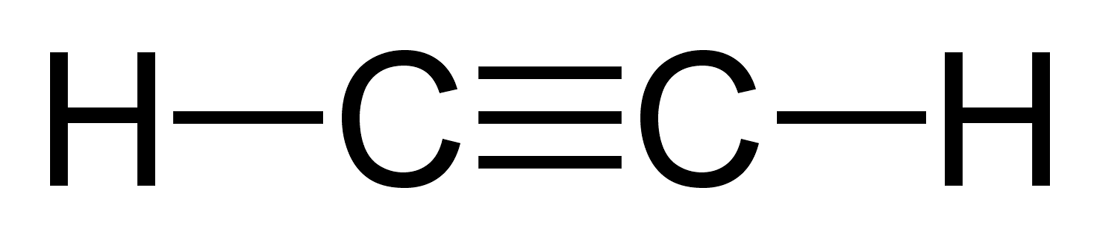
Chemical Formula for Acetylene (or Ethyne)
An acetylene molecule has two carbon atoms bonded by a triple bond and two hydrogen atoms. The orientation is in the same straight line and the bond angle is 180 degrees. The chemical formula for acetylene in written as-
| Acetylene = C2H2 |
Acetylene and other alkynes are generally prepared from calcium carbide, vicinal dihalides or by dehydrohalogenation of alkyl dihalides. Check out the complete preparation of alkynes from this article.
To get more such chemical formulas and other related articles, keep visiting BYJU’S.
Comments