What is Acid Dissociation in Water?
Solvated hydroxide and solvated protons are in equilibrium with liquid water. To define the concentration of these solvated ions in water, we usually use a value related to the equilibrium constant. The Kw constant for water dissociation is 1 x 10-14.
For acidic and basic solutions, pH and pOH are critical values. The log base 10 of the hydrogen ion concentration or the log base 10 of the hydroxide ion concentration, respectively.
pH = -log [H+]
pOH = -log [HO–]
14 = pH + pOH
The LeChateliers principle states that adding one of the products to an equilibrium system causes the equilibrium to shift towards reactants. Acids and bases dissolve in water and suppress water dissociation by increasing the concentration of one of the products of water self-ionization, either protons or hydroxide ions.
Acid and base solutions in water are typically described using pH and pOH. The concentration of solvated protons equals that of solvated hydroxide anions in pure water, and the pH is 7. The pH of acidic solutions is lower, whereas the pH of basic solutions is higher.
Table of Contents
- Definitions of Acid Dissociation Constant
- Strong Acids and Water Dissociation
- Weak Acids and Water Dissociation
- Acidity in nonaqueous solutions
- Frequently Asked Questions
Definitions of Acid Dissociation Constant (Ka)
The acid dissociation constant is a direct result of the dissociation reaction’s underlying thermodynamics; the pKa value is proportional to the standard Gibbs free energy change for the reaction. The pKa value fluctuates with temperature and may be understood qualitatively using Le Châtelier’s principle: when the reaction is endothermic, Ka increases and pKa drops with increasing temperature; when the reaction is exothermic, Ka increases and pKa reduces with increasing temperature.
An acid is a chemical that dissociates in an aqueous solution, producing the hydrogen ion H+ (a proton), according to Arrhenius’s original molecular definition:
HA ⇌ A– + H+
H2O ⇌ H+ + OH–
A dissociation constant is the equilibrium constant for this dissociation reaction. Because the released proton reacts with a water molecule to form the hydronium (or oxonium) ion H3O+ (uncovered protons don’t exist in solution), Arrhenius proposed that the dissociation be represented as an acid-base reaction:
HA + H2O ⇌ A– + H3O+.
This was further generalised by Bronsted and Lowry to a proton exchange reaction:
Acid + Base ⇌ Conjugate base + Conjugate acid.
A proton is lost from the acid, leaving a conjugate base; the proton is transferred to the base, forming a conjugate acid.
Read More:
Strong Acids and Water Dissociation
In an aqueous solution, strong acids dissociate fully and have negative Ka values. We can assume that the [H+] in a strong acid solution is equal to the acid’s initial concentration. The following is a list of strong acids.
AH + H2O → A– + H+
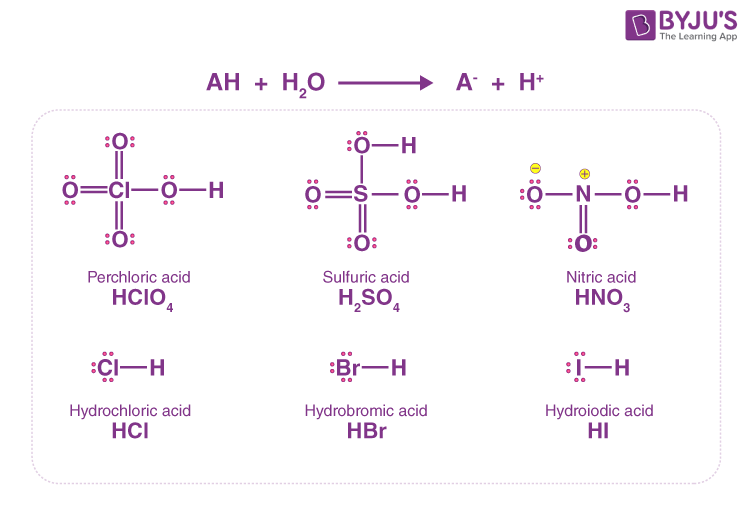
Weak Acids and Water Dissociation
For weak acids, there is an equivalent situation where the [H+] due to acid dissociation is comparable to the [H+] due to water dissociation (1.0×10-7 M). Water dissociation can contribute to the pH of a weak acid solution if the acid is extremely dilute, very weak, or dilute and weak, unlike strong acids, where it is important only when a very dilute acid is involved.
In an aqueous solution, weak acids dissociate only partially. The pKa = -log (Ka). The table below shows some of the weak acids:
| Acid | Reaction | pKa |
|---|---|---|
| Hydrofluoric acid | HF ⇌ H+ + F– | 3.17 |
| Carbonic acid | H2CO3 ⇌ H+ + HCO3– | 6.37 |
| Bicarbonate | HCO3– ⇌ H+ + CO3-2 | 10.25 |
| Bisulfate | HSO4– ⇌ H+ + SO4-2 | 1.99 |
| Ammonium | NH4+ ⇌ H+ + NH3 | 9.24 |
| Hydrogen sulfide | H2S ⇌ H+ + HS– | 7.0 |
| Water | H2O ⇌ H+ + HO– | 15.74 |
Acidity in nonaqueous solutions
In the following situations, a solvent is more likely to cause ionisation of a dissolved acidic molecule:
- It’s a protic solvent, which means it can create hydrogen bonds.
- It has a large donor base, indicating that it is a powerful Lewis base.
- Because of its high dielectric constant (relative permittivity), it is an excellent ionic species solvent.
The aprotic solvents dimethyl sulfoxide (DMSO) and acetonitrile are frequently used to determine the pKa values of organic compounds (ACN).
In an acidic solvent, acid ionisation is less than in water. When hydrogen chloride is dissolved in acetic acid, for example, it becomes a weak acid. Because acetic acid is a far weaker base than water, this is the case.
HCl + CH3CO2H ⇌ Cl– + CH3C+(OH)2
acid + base ⇌ conjugate base + conjugate acid
Frequently Asked Questions on Acid Dissociation
What happens when acids dissociate in water?
An acid dissociates into hydrogen ions (H+) and anions in an aqueous (watery) solution. A strong acid’s molecules dissociate, resulting in a large concentration of H+.
What is a protic solvent?
A protic solvent has a hydrogen atom bound to an oxygen (as in a hydroxyl group), nitrogen (as in an amine group), or fluoride (as in a fluoride group) (as in hydrogen fluoride). Any solvent that contains a labile H+ is referred to as a protic solvent. Solvent molecules rapidly supply protons (H+) to solutes, commonly through hydrogen bonding. The most frequent protic solvent is water.
In basic terms, what is the equilibrium constant?
The relationship between the number of products and reactants present at equilibrium in a reversible chemical reaction at a particular temperature is expressed by this number.
What do the terms Lewis acid and Lewis base mean?
Lewis acids and bases are electron-pair acceptors and electron-pair donors, respectively. As a result, a Lewis base can give a pair of electrons to a Lewis acid, resulting in a product with a coordinate covalent bond. A Lewis adduct is another name for this item.
What is meant by dielectric constant (relative permittivity)?
A solvent’s polarity is determined by its dielectric constant (symbol: ℇ). The higher a solvent’s dielectric constant, the more polar it is. Water has a larger dielectric constant than methanol, indicating that it is more polar. A covalent solute dissociates into ions to a larger extent in water than in methanol, which has practical implications.

Comments