What is an Addition Reaction?
In the simplest of terms of organic chemistry, we can say that an addition reaction is a chemical reaction wherein two or more reactants come together to form a larger single product.
Table of Contents
- Addition Reaction Explanation
- Types of Addition Reactions
- Electrophilic addition
- Nucleophilic addition
- Frequently Asked Questions – FAQs
Addition Reaction Explanation
But only chemical compounds containing multiple bond character can undergo an addition reaction as a double or triple bond is usually broken to form the required single bonds. An addition reaction is essentially a reverse of a decomposition reaction wherein a decomposition reaction is a reaction where one compound decomposes into one or more elements or compounds. Looking at an example of an addition reaction, hydrochlorination of propane (an alkene), for which the equation is
CH3CH = CH2 + HCl → CH3C+HCH3 + Cl− → CH3CHClCH3
Types of Addition Reactions
For polar addition reactions there are two classifications, namely:
- Electrophilic Addition reactions
- Nucleophilic Addition reactions
For non-polar addition reactions, we have two classifications, namely:
- Free radical addition reactions
- Cycloadditions reactions
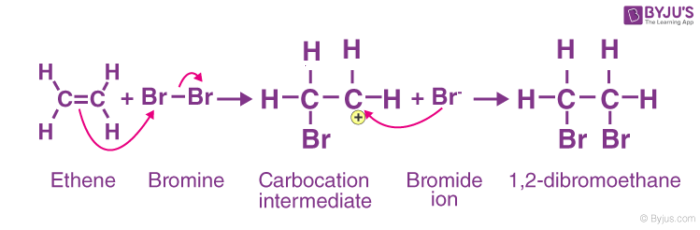
Electrophilic addition:
An electrophilic addition reaction can be described as an addition reaction in which a reactant with multiple bonds as in a double or triple bond undergoes its π bond broken and two new σ bonds are formed.
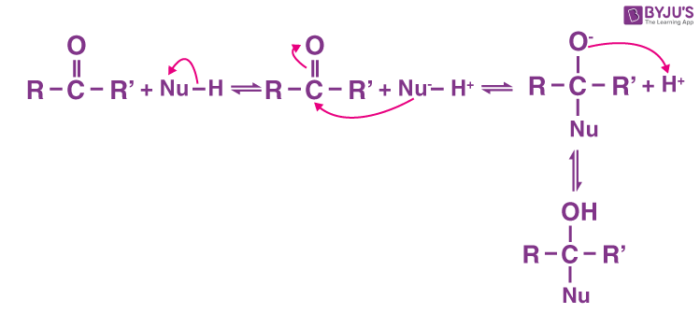
Nucleophilic addition:
A nucleophilic addition reaction is an addition reaction where a chemical compound with an electron-deficient or electrophilic double or triple bond, a π bond, reacts with a nucleophile which is an electron-rich reactant with the disappearance of the double bond and creation of two new single, or σ, bonds.
Recommended Videos
Hydrohalogenation of alkynes

Frequently Asked Questions – FAQs
What is the main feature of addition reaction?
When an atom is added to a combination with a double or triple bond, an addition reaction occurs. Addition reactions are linked to unsaturated molecules. These are hydrocarbons with two or three double or triple bonds. After an addition reaction is completed, there are no reactant residues left.
What causes addition reaction?
In organic chemistry, an addition reaction is an organic reaction in which two or more molecules combine to generate a bigger one (the adduct). Molecules with carbon—hetero double bonds, such as carbonyl (C=O) or imine (C=N) groups, can be added because they have double-bond character as well.
Is halogenation an addition reaction?
A halogen addition reaction is a simple organic reaction in which a halogen molecule is added to an alkene functional group’s carbon–carbon double bond. This reaction is a combination of halogenation and electrophilic addition.
Is hydration an addition reaction?
In the presence of a catalyst, alkenes undergo an addition reaction with water to create alcohol. Hydration is the name for this sort of addition reaction. Water is directly injected into the carbon–carbon double bond.
What is photo halogenation reaction?
A haloalkane is formed when a halogen reacts with an alkane in the presence of ultraviolet (UV) radiation or heat (alkyl halide). The chlorination of methane is one example. This phenomenon is explained by the reaction mechanism. Mechanism of halogenation.


Thank you 😊
very useful
thanks for giving suitable example of electrophilic and nucleophilic addition