What is Aluminium Hydroxide?
Al(OH)3 is amphoteric in nature with the chemical name Aluminium hydroxide.
Aluminium hydroxide is also called Aluminic acid or Aluminic hydroxide or Aluminium (III) hydroxide. It is found in nature in the form of mineral gibbsite and its polymorphs viz doyleite, nordstrandite, and bayerite.
Aluminic hydroxide is an amorphous powder white. It is insoluble in water but soluble in alkaline and acidic solutions.
Table of Contents
- Aluminium Hydroxide Structure
- Properties of Aluminium hydroxide
- Production of Aluminium hydroxide
- Uses of Aluminium hydroxide
- Health Hazards for Aluminium hydroxide
- FAQs
Aluminium Hydroxide Structure – Al(OH)3
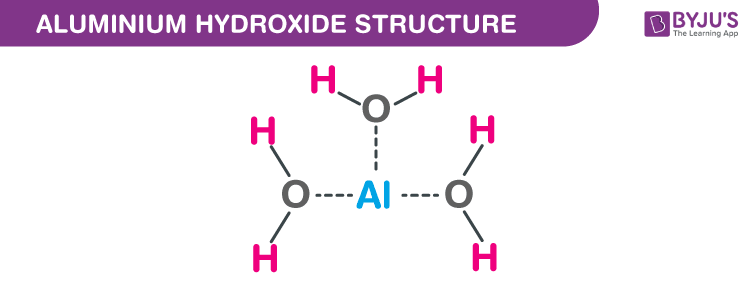
Aluminium hydroxide structure – Al(OH)3
Properties of Aluminium hydroxide – Al(OH)3
| Al(OH)3 | Aluminium hydroxide |
| Molecular weight of Al(OH)3 | 78.00 g/mol |
| Density of Aluminium hydroxide | 2.42 g/dm3 |
| Flashpoint of Aluminium hydroxide | Non-flammable |
| Melting Point of Aluminium hydroxide | 300 °C |
Aluminic hydroxide has a typical structure of metal hydroxide consisting of hydrogen bonds. It comprises double layers of hydroxyl groups along with aluminium ions which occupy 2/3rd of the octahedral holes which are formed between the two layers.
Gibbsite is amphoteric and acts as a Brønsted-Lowry base to yield a salt by picking up hydrogen ions and neutralizing the acid. The reaction is as follows:
3HCl + Al(OH)3 → AlCl3 + 3H2O
It acts as a Lewis acid in bases. It takes away an electron pair from the hydroxide ions. The re4action is as follows:
Production of Aluminium hydroxide
Commercially used aluminium hydroxide is manufactured by the Bayer process. It is carried out by dissolving bauxite in sodium hydroxide solution at a temperature range up to 270 °C. The waste is removed and the sodium aluminate solution is allowed to precipitate. Therefore, the precipitate obtained is aluminium hydroxide. Alumina or aluminium oxide can be obtained from aluminium hydroxide by the process of calcination.
Bayer Process
Step-1 : Al2O3.2H2O + 2 NaOH → 2 NaAlO2 + 3 H2O
Step-2 : NaAlO2 + 2 H2O → Al(OH)3 + NaOH
Step-3 : 2 Al(OH)3 → Al2O3 + 3 H2O
Al(OH)3 Uses (Aluminium hydroxide)
- Aluminium hydroxide is used as a flame retardant in plastics.
- Used as an antacid.
- Used in aluminium Hydroxide gel.
- Used to manufacture activated alumina.
- Used as a filler in cosmetics.
- Used as a chemical intermediate.
- Used as a soft abrasive for plastics.
- Used in glass additive to increase resistance to thermal shock.
- Used in waterproofing fabrics.
- Used in the manufacturing of glass.
Health Hazards
Prolonged exposure to Aluminium(III) hydroxide it causes irritation in eyes, respiratory system, and skin. When comes in contact with water it causes a violent explosion.
Frequently Asked Questions – FAQs
What does aluminium hydroxide do?
Aluminium is a metal that occurs naturally. The antacid is the hydroxide of aluminium. Aluminium hydroxide is used in the treatment of heartburn, stomach pain, sore stomach or indigestion with acid. Aluminium hydroxide is also used in humans with other kidney disorders to reduce phosphate levels.
Is Aluminium hydroxide safe in cosmetics?
Hydroxide in aluminium. The synthetic ingredient acts as an opacifier. Primary applications include agents and absorbents for painting. There is no known skin toxicity to the aluminium hydroxide.
What is another name for aluminium hydroxide?
Aluminium hydroxide is an over-the-counter antacid drug used to treat peptic ulcers and hyperphosphatemia.
Is aluminium hydroxide a weak base?
Aluminium hydroxide has the molecular formula Al(OH)3 as a chemical compound. … For example, in aluminium hydroxide, the hydroxide (OH) can act as a weak base when reacting with the strong acid, hydrochloric acid (HCl). A weak base is a base that partially dissociates in solution, or breaks apart.
Is aluminium hydroxide dangerous?
Side effects of aluminium hydroxide include intense stomach pain or constipation, lack of appetite; discomfort while urinating; muscle weakness, fatigue; extreme drowsiness.


Comments