Ammonium chloride formula is given in the following points. It is first important to recall that ammonium chloride is an inorganic compound. It is a white crystalline salt and is easily soluble in water. Check the ammonium chloride chemical formula (or molecular formula) with its structural formula below.
Structural Formula of Ammonium Chloride
Ammonium chloride is a water-soluble and mildly acidic inorganic compound. It has a nitrogen ion, four hydrogen atoms, and a chloride ion. The structure of ammonium chloride can be represented as follows.
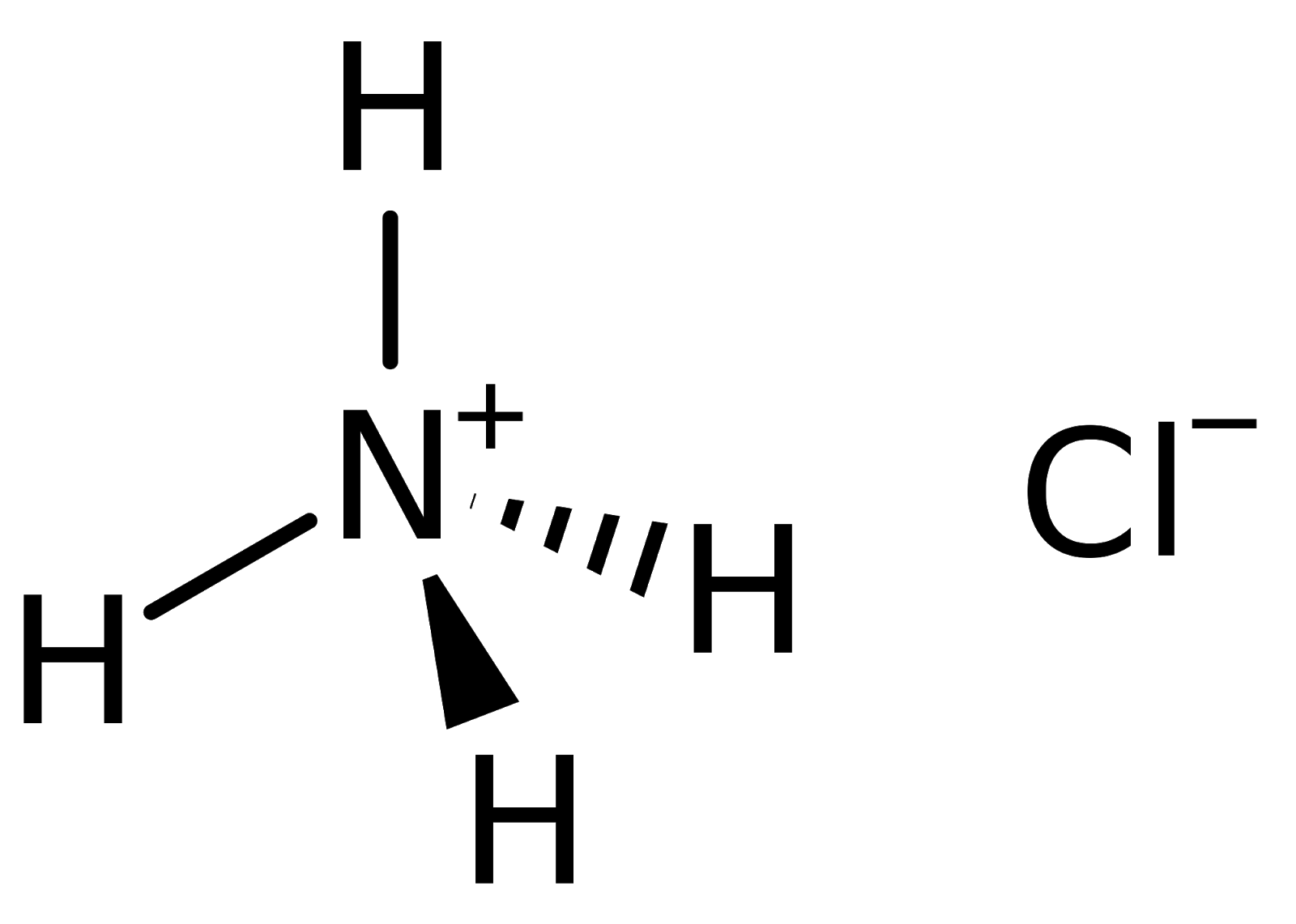
Chemical Formula of Ammonium Chloride
The ammonium chloride molecule consists of nitrogen, chloride and hydrogen atoms. Learn more about ammonium chloride here along with its complete structure, properties, and uses in the linked article. The chemical or molecular formula for ammonium chloride is written as follows-
| Ammonium Chloride Chemical Formula= NH4Cl |
Ammonium chloride is used in several sectors like in fertilizers, glue, medicines, food additives, cooling baths, and as an acidifier. There are various other uses of NH4Cl but its use should be limited due to the environmental hazards it poses.
To learn formulas of several other chemical compounds and to learn various other chemistry topics in a more efficient way, stay tuned with BYJU’S and register now.
Comments