What is Argon Gas?
Argon is the first noble gas to be discovered. It was identified by the English physicist Lord Rayleigh and Scottish chemist William Ramsay in 1894. Argon is from the Greek word “Argos” which means “lazy” or “inactive”. It belongs to noble gas and makes up about 0.93% of Earth’s atmosphere. It is the third most abundant gas in the atmosphere. Non-flammable cryogenics gases are sometimes referred to as inert gases.
| Ar | Argon |
| Density | 1.784 g/L |
| Molecular Weight/ Molar Mass | 39.948 u |
| Boiling Point | -185.8 °C |
| Melting Point | -189.4 °C |
| Chemical Formula | Ar |
Argon Gas Structure – Ar
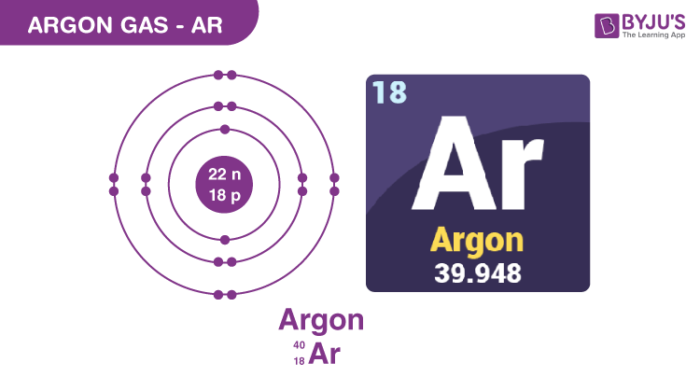
Physical Properties of Argon Gas – Ar
| Odour | Odourless |
| Appearance | Colourless gas |
| Period (periodic table) | period 3 |
| Block (periodic table) | p-block |
| Atomic number (Z) | 18 |
| Element category | Noble gas |
| Solubility in water | 62 mg/L at 20oC and 1 bar pressure |
Chemical Properties of Argon Gas- Ar
Argon is a noble gas that is chemically inert but at low temperature, it is possible to combine with other atoms to form very fragile compounds which exist at very low temperatures. Since this element does not exhibit any chemical reactivity it is called a noble gas.
Uses of Argon Gas – Ar
- Argon-enhanced electrosurgery uses argon gas to increase the effectiveness of the ESU, resulting in less tissue damage and less blood loss.
- Argon plasma coagulation uses electricity conductive argon plasma as a medium to deliver a high frequency current to coagulate tissue. The noncontact feature permits rapid coagulation with minimal manipulation and trauma to the target tissue.
- In the electric lamp industry, argon is used as the standard filling for incandescent filament lamps and neon discharge tubes being familiar in advertising signs.
Frequently Asked Questions
What are the uses of argon gas?
Argon gas is widely used for filling up incandescent and fluorescent light bulbs in order to prevent oxygen from corroding the filament of the bulb (which usually gets heated to very high temperatures). Argon is also used to form inert atmospheres that require shielding from other atmospheric gases for arc welding, rising semiconductor crystals, and other processes.
How is argon produced?
Industrially, argon gas is produced via the fractional distillation of liquid air in a cryogenic air separation unit, This procedure separates liquid nitrogen (boiling point: 77.3 K) from argon (boiling point: 87.3 K) and liquid oxygen (boiling point: 90.2 K). Every year about 700,000 tons of argon is produced around the world.
Is argon gas toxic?
Despite the fact that argon gas is non-toxic, in closed areas it is 38 per cent denser than air and it, therefore, can be considered as a dangerous asphyxiant. It’s hard to spot because it’s colourless, odourless, and without taste.

Comments