What is Barium nitrate?
Ba(NO3)2 is an inorganic compound with chemical name Barium nitrate. It is also called Barium dinitrate or Nitrobarite or Barium salt. It is a good oxidizing agent, burns with a green coloured flame. It is widely used in pyrotechnics.
Nitrobarite is a crystalline solid white in colour. It is a non-combustible compound, but enhances the burning of combustible elements. When exposed to fire or heat for a long duration it may explode.
Properties of Barium nitrate – Ba(NO3)2
| Ba(NO3)2 | Barium nitrate |
| Molecular weight of Ba(NO3)2 | 261.337 g/mol |
| Density of Barium nitrate | 3.24 g/dm3 |
| Refractive index | 1.5659 |
| Melting Point of Barium nitrate | 592 °C |
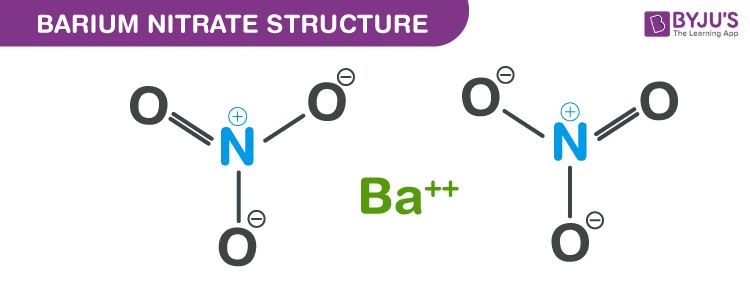
Barium nitrate structure – Ba(NO3)2
- Barium nitrate is used in the production of barium oxide containing materials.
- Used in green signal lights.
- Used in making ceramic glazes.
- Used as a rodenticide.
- Used in detonators.
- Used in primers and tracer bullets.
- Used in the making of paints.
- Used as an oxidizing agent.
- Used in making explosives.
- Used as a propellent and blowing agent.
Production of Barium nitrate
Barium dinitrate is produced by two processes that start with the source component for barium viz carbonate.
Method 1:
- Dissolve barium carbonate (BaCO3) in nitric acid (HNO3)
- Allow the precipitation of iron impurities
- Separate the impurities by filtration
- Evaporation
- Crystallization.
Method 2:
It is obtained by combining barium sulfide (BaS) with nitric acid (HNO3). At higher temperatures, barium nitrate (Ba(NO3)2) decomposes to barium oxide (BaO):
2Ba(NO3)2 → 2BaO + 4NO2 + O2
Health hazards
Inhaling or contact of Nitrobarite with skin or eyes can cause irritation. Swallowing results in excessive salivation, colic, convulsive tremors, elevated blood pressure, vomiting, diarrhea, and hard pulse. It may also lead to hemorrhages in the intestines, kidneys, and stomach. Also, muscular paralysis may occur.
Frequently Asked Questions
What are the uses of barium nitrate?
Barium nitrate is used in the manufacture of substances containing barium oxide. This compound is also used in the barium oxide production process, in the vacuum tube industry and in pyrotechnics for green fire. Barium nitrate has also been a key ingredient in the explosive charge “SR 365”.
What happens when barium nitrate is heated?
When burned, barium nitrate emits a green flame. It decomposes to barium oxide, oxygen, and nitrogen dioxide when heated. This converts barium nitrite into nitric oxide. To produce barium sulfate it combines with either sulfate or sulfuric acid.
Learn more about the Structure, physical and chemical properties of Ba(NO3)2 from the experts at BYJU’S.


Comments