Bromine exists as a diatomic molecule with the chemical formula Br2 that belongs to the halogen group. Bromine has only one Br-Br bond in its Lewis structure, and each bromine atom has three lone pairs. There is a single bond between the bromine atoms and three lone pairs between the bromine atoms.
Bromine is the third lightest atom of the halogens and exists as both a reddish-brown liquid and a reddish-brown gas at normal room temperature. Since the elemental Bromide is extremely reactive, it does not materialise freely in nature. It is available as a soluble colourless halide crystalline mineral salt, similar to table salt.
Like the other halogens, its lack of one electron in forming an octet which makes it a strong oxidising agent. Thus, it reacts with various elements to complete its octet in the outermost shell and to achieve stability.
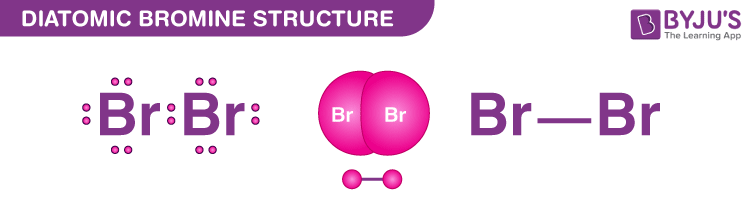
Table of Contents
- How to draw Lewis Structure for Bromine
- Molecular Geometry of Bromine
- Hybridisation of Bromine
- Polarity of Bromine
- Frequently Asked Questions – FAQs
How to draw Lewis Structure for Bromine
There are a few steps that need to be followed to attain the stable and correct Lewis structure which are as follows:
1. Determine the total number of electrons in the valence shells of bromine atoms.
The bromine molecule contains only one element. In the periodic table, bromine is a group VIIA element with seven electrons in its last shell.
Therefore, the total number of valence electrons = 7(2) = 14.
2. Total electron pairs exist in the form of lone pairs and bonds.
Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the Br2 molecule, there are a total of seven pairs of electrons.
3. Determine the central atom.
There are only two atoms and they both belong to the same element, therefore, the central atom will be bromine only.
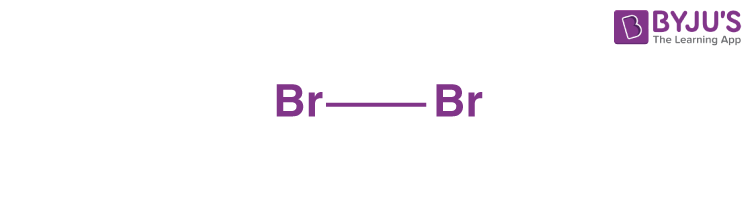
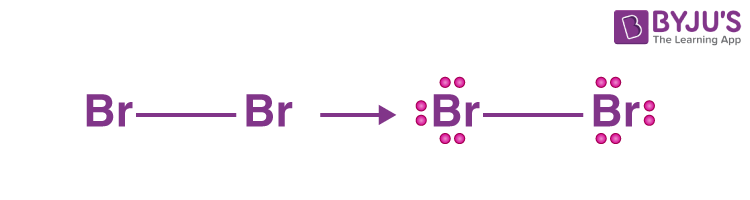
4. Mark atoms with lone pairs.
- There are a total of seven electron pairs.
- There is already one bond in the drawn structure. As a result, there are six more lone pairs on bromine atoms to mark.
- We can mark the remaining lone pairs on both bromine atoms because there is no central atom.
- Each bromine atom will take three lone pairs.
- All valence electron pairs have now been marked.
5. If there are charges, mark them.
There are no charges in the molecule of bromine.
6. To obtain the best Lewis structure, check the stability and minimise charges on atoms by converting lone pairs to bonds.
It can be checked by using the formula-
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
| Element | Bromine |
|---|---|
| Formula Applied | Valence electrons = 7
Lone pair electrons = 6 Shared pair electrons (1 single bond) = 2 |
| Formal Charge | (7 – 6 – 2/2) = 0 |
Since the overall formal charge is zero, the above Lewis structure of Bromine (Br2)is most appropriate, reliable, and stable in nature.

Molecular Geometry of Bromine
Both Bromine atoms have 7 electrons in their outermost valence shell in the case of Dibromine, or Br2. To attain stability and complete the octet, two Bromine atoms combine together.
Dibromine’s molecular geometry is linear due to the presence of atoms of the same element, the compound has a symmetrical shape, and the bond angle is 180 degrees.
Hybridisation of Bromine
The general formula for calculating a compound’s hybridisation is:
Hybridisation of a molecule = No. of sigma bonds + No. of lone pairs
The Br2 molecule contains three lone pairs and one sigma bond.
As a result, the hybridisation of Br2 is sp3. It has a linear structure.
Polarity of Bromine
Br2 (Bromine) is nonpolar because both bromine atoms in this molecule have the same electronegativity, resulting in equal charge distribution and a net-zero dipole moment.
Frequently Asked Questions on
Why does bromine have 7 valence electrons?
Valence electrons are found in the highest energy s and p orbitals. Bromine has an electron configuration of 1s22s22p63s23p64s23d104p5 with the valence electrons in the 4s and 4p orbitals, giving it 7 valence electrons.
How many dots should there be in a bromine atom’s Lewis symbol?
In Lewis electron dot diagram of Bromine atom, there should be seven dots arranged correctly. As it has seven valence electrons.

Why is Br2 classified as a nonpolar molecule?
The bromine molecule has a geometrical structure that is linear. Additionally, it contains two bromine atoms. When two atoms in a molecule have the same electronegativity value, they have the same influence on bonded electrons. As a result, both atoms have an equal charge distribution.
What is the hybridisation and electron and molecular geometry of Br2?
The hybridisation of bromine molecule is sp3 and electron and molecular geometry of Br2 is linear with the bond angle 180°.
What type of bond is present in the molecule of bromine?
The bromine atoms are bonded by the single covalent bond in the bromine molecule.
Comments