
What is Carbon Dating?
Carbon dating is one of the archaeology’s mainstream methods for dating organic objects up to 50,000 years old. This method is based on the idea of radiative decay of Carbon-14 isotopes over thousands of years. Through physics, scientists have discovered that radioactive molecules decay at a specific rate dependent on the atomic number and mass of the decaying atoms. This constant can be used to determine the approximate age of the decaying material through the ratio of radioactive isotopes to the estimated initial concentration of these isotopes at the time of the organism’s death. Scientists have concluded that very little change has occurred in the ratio of Carbon-12 to Carbon-14 isotopes in the atmosphere meaning that the relationship between these two should be very similar to how they remain today.
Why is it important?
Without radiocarbon dating, “we would still be foundering in a sea of impressions sometime bred of inspired guesswork, but more often of imaginative speculation”. Carbon-14 dating is a revolutionary advancement in the study of the history of our planet. It is, in fact, leading to the “reconstruction of the history of the world”. This method of dating allows researchers to learn about past civilizations, changes in the earth, and in the climate.
Different civilizations and religions have different methods of dating. However, carbon-14 dating offers something particularly valuable, called absolute dating, which is the age of the substance before the current time. This means that it may be used and compared to dates anywhere in the world. In fact, it is considered the, “most important development in absolute dating in archaeology and remains the main tool for dating the past 50,000 years”. With this tool, scientist hopes to unravel the mysteries of how man developed, when the first man lived, where he went, and create a type of timetable of human life.
Physics of Carbon Dating
Carbon has unique properties that are essential for life on earth. Familiar to us as the black substance in charred wood, as diamonds, and the graphite in “lead” pencils, carbon comes in several forms, or isotopes. One less abundant form of carbon has atoms that are 14 times as heavy as hydrogen atoms: carbon-14, or 14C, or radiocarbon.
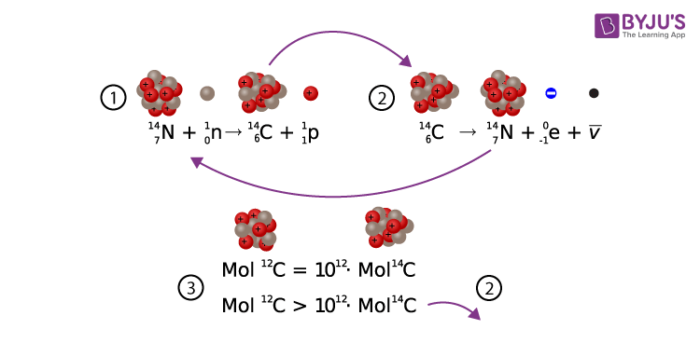
Carbon-14 is a radioactive substance. At any given moment carbon-14 is decaying in an object, and if that object is living, it is also being replaced at a steady rate. Carbon- 14 is created when a neutron is excited by a cosmic ray, and then that neutron collides with a nitrogen atom. The carbon isotope is when absorbed by plants through photosynthesis and consumed by animals. Due to the way the sunlight reacts with the atmosphere, it is also taken in by respiration.

Comments