Table of Contents
- What is a Carboxyl Group?
- The formula of Carboxyl Group
- Structure
- Properties
- Preparation of Acetic and Benzoic Acid
What is a Carboxyl Group?
Carboxyl groups are a combination of two functional groups attached to a single carbon atom, namely, hydroxyl (single-bonded OH) and carbonyl (double bonded O) groups. The carboxyl (COOH) group is so-named because of the carbonyl group (C=O) and a hydroxyl group. They include carboxylic acids and amino acids. In the IUPAC system, these have the suffix -oic acid.
The formula of Carboxyl group
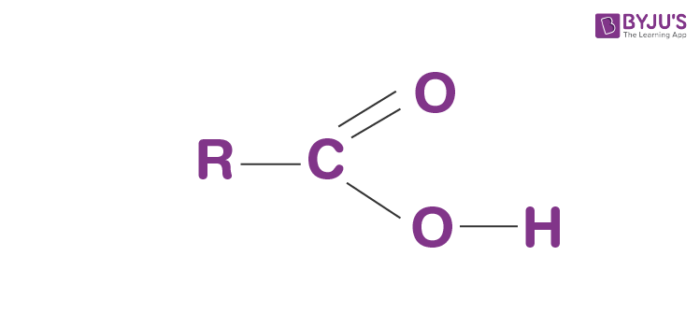
The formula for the carboxyl group is R-COOH wherein R is the organic compound chain.
Structure of Carboxyl group
Carboxyl groups are present at the end of the chain. When it ionises, it releases a proton (H+) and negatively charged oxygen. This charge turns about forward and backwards between the 2 oxygen molecules, which makes this ionized state moderately steady. The carboxylate ion formed is extremely stable due to resonance.
Properties of Carboxyl Group
- In the carboxylic acid group, carbon is attached to a highly electronegative oxygen, making the bond polar.
- A compound comprising a carboxyl group will possess a high melting point, hydrophilic centres, and boiling point.
- The reason behind high melting and boiling point can be dissipated because of the formation of a hydrogen bond in the solid and liquid state. One of the typical examples is fatty acids.
- Earlier members of carboxylic acids are soluble in water due to hydrogen bonding. Higher members have less solubility due to the increasing hydrophobic nature of the carbon chain.
Preparation of Acetic Acid and Benzoic Acid
Acetic acid can be naturally blended either by anaerobic fermentation, the procedure used to make vinegar. The rigorous method requires warm ethanol and oxygen with Acetobacter. The anaerobic process requires just sugar as an input chemical, and acetogen can then give carboxylic acids as yield. It ought to be noticed that the high-impact process is still predominantly connected due to acetogenins utilized for anaerobic procedures indicating less resistance to acidic situations. At the end of the day, acetogenesis will be destroyed if a lot of acids are produced to a great volume.
Benzoic acid is a fundamental part of benzoin resin. It is genuinely expensive to remove benzoic acid specifically from benzoin resins. Along these lines, most of the benzoic acid in the market is produced mechanically.
Register with BYJU’S for more learning content.


Comments