What is the Melting Point?
The melting point is usually defined as the point at which materials changes from a solid to a liquid.
The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. This is the point at which both liquid and solid phase exists at equilibrium. The melting point of the substance also varies with pressure and is specified at standard pressure.
Table of Content
- What is the boiling point?
- Boiling Point Definition
- Boiling Point of Water
- Recommended Videos
- Melting Point Determination Principle
- Melting and Boiling Points of Some Elements
- Mixed Melting Points
- FAQs
The term ‘freezing point’ is used to denote the temperature at which a liquid is converted into a solid and can, therefore, be viewed as the opposite of the term ‘melting point’. However, substances can be cooled below their freezing points without the formation of a solid. Such liquids are known as supercooled liquids.
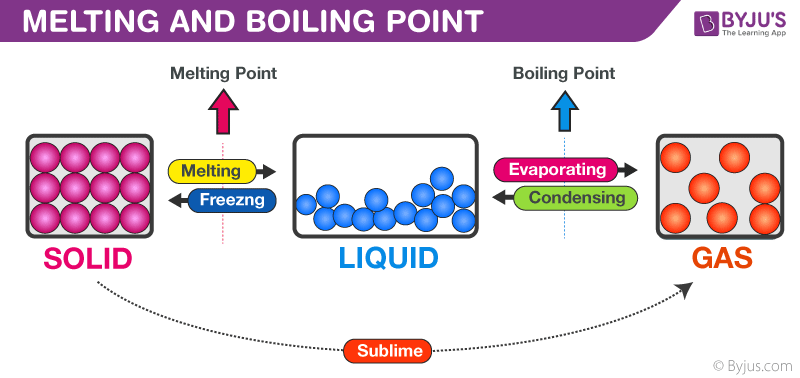
Melting Point and Boiling Point
What is Boiling Point?
The boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure of the liquid’s environment. At this temperature, the liquid is converted into a vapour.
The boiling point of the liquid depends upon the pressure of the surrounding. When the liquid is at high pressure, it has a higher boiling point than the boiling point at normal atmospheric pressure. The boiling point of different liquids is different for a given pressure. In 1982, IUPAC defined the standard boiling point of a liquid as the temperature at which the liquid boils under a pressure of 1 bar.
Boiling Point Definition
The temperature at which liquid vapour pressure equals atmospheric pressure is referred to as boiling point. The boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure surrounding it.
The boiling point for any material is the temperature point at which the material transforms into the gas phase in the liquid phase. This happens at 100 degrees centigrade for water. The Celsius scale was in fact created on the basis of the ice/water melting point and the liquid water/vapor boiling point. Each substance carries its own boiling point.
The boiling point of a substance is dependent on the pressure of its surroundings. In mountainous terrains (where the altitude is high), the pressure of the atmosphere is relatively lower than the atmospheric pressure at sea level. This is the reason why food cooks at a slower pace in mountainous areas (the lower atmospheric pressure causes water to boil at temperatures below 100oC).
When all the particles in the liquid phase have been transformed into the gas phase, the temperature begins to rise again, as long as heat is still being applied to the surrounding system. As the temperature starts to increase, so does the particle’s kinetic energy.
Boiling Point of Water
Water can boil, raise temperature or decrease air pressure, in two ways. At sea level, it is the pressure of air that causes water to boil at 100oC. Water can boil at a much lower temperature in vacuum, where there’s no air. That is, if not for the skin that keeps the blood pressurized, body temperature would be sufficient to cause the blood to boil with water. At low air pressure the water boils significantly below 100oC at temperatures.
The boiling point of water is the temperature at which the liquid water vapor pressure is equal to the pressure surrounding the body, and the body transforms into a vapour. The boiling point is the temperature for a particular liquid to boil at. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees Celsius. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure.
Recommended Videos

Melting Point Determination Principle
- Melting point may be defined in various ways, each corresponding to a different residual amount of solid fat.
- The capillary tube melting point, also known as the complete melting point or clear point, is the temperature at which fat heated at a given rate becomes completely clear and liquid in a one end closed capillary.
- The slip melting point is performed similarly to the capillary tube method and measures the temperature at which a column of fat maves in an open capillary when heated.
- The dropping melting point or dropping point is the temperature at which the sample flows through a 0.11-in hole in a sample up placed in a specialized furnace.
- The Wiley melting point measures the temperature at which a 1/8 x 3/8 in disc of fat suspended in an alcohol water mixture of similar density changes into a sphere.
Melting Point Determination
The melting point is determined in a capillary tube. The temperature at which the substance is completely melted as indicated by the disappearance of the solid, will be in the range of + or – 4oC from the stated value, unless otherwise indicated.
Details of the Procedure
The following technique is adequate for the determination of melting point.
Grind about 50mg of the substance to be tested in a small motor. Place the ground substance in a vacuum desiccator over silica gel or phosphorus pentoxide at room temperature and dry for about 24 hours. Place the substance in a dry capillary tube of 1mm internal diameter forming a column about 3mm high. Heat the melting point apparatus to a temperature of 5-10oC below the expected temperature of melting and adjust the heating so that the temperature in the chamber rises about 1oC per minute. Introduce the capillary with the substance into the heated chamber and note the temperature when the sintered substance becomes completely transparent, this is considered to be the melting point.

Melting Point Determination
The melting point denotes the temperature at which the substance has just completely melted; this is indicated by the disappearance of the solid phase and complete transparency of the melt.
Melting and Boiling Points of Some Elements
A tabular column listing the melting points and boiling points of some important elements is provided below.
| Name of the substance | Boiling point(K) | Melting point(K) |
| Aluminium | 2740 | 932 |
| Copper | 1460 | 1359 |
| Gold | 2933 | 1336 |
| Hydrogen | 20.3 | 13.8 |
| Mercury | 630 | 234 |
Mixed Melting Points
In the majority of cases, the presence of a foreign substance will lower the melting point of a pure organic compound. This fact is utilised in the so-called mixed melting point test for the identification of organic compounds.
Consider an organic compound X having a melting point of 140oC is suspected to be o-chlorobenzoic acid. Its identity may be established by performing a melting point determination of a mixture containing approximately equal weights of X and of an authentic specimen of o-chlorobenzoic acid (A). If the melting point of the mixture is 140oC then X is o-chlorobenzoic acid, but if the melting point is depressed by several degrees A and X cannot be identical. It is recommended that at least three minutes containing say 20 percent X +80 percent A:50 percent X+50 percent A; and 80 percent X+20 percent A be prepared and the melting point be determined.
Frequently Asked Questions – FAQs
What is the melting point?
The temperature at which a solid becomes a liquid due to enough heat. For a given substance, its solid form’s melting point is the same as its liquid form’s freezing point and depends on factors such as the substance’s purity and surrounding pressure.
What increases the melting point?
A compound’s melting point is determined by the force of attraction between molecules and symmetry. Higher melting points result from stronger intermolecular interactions. Ionic compounds usually have high melting points because the ion-ion interaction of the electrostatic forces is much stronger.
Are melting and freezing points the same?
To sum up, as matter transforms from solid to liquid (melting) or liquid to solid (freezing), its temperature is set at the same temperature as the melting/freezing point.
What determines the boiling point?
A molecule’s boiling point is based on its structure. Check for the difference in functional molecule groups when determining the boiling point of molecules of similar size. Ethers have a lower boiling point than alkanes because they have dispersion powers in London and interactions between the dipole and the dipole.
Why is the boiling point important?
Organic compounds ‘ boiling point can provide important information regarding their physical properties and structural characteristics. The boiling point helps to identify a compound and to characterize it. Higher-Pressure water has a higher boiling point than when the atmospheric pressure is lower.
Why is boiling point important?
The boiling point of organic compounds will provide valuable details about their physical and structural properties. Boiling point assists in the recognition and characterization of a compound. A liquid at higher pressure has a higher boiling point than when the atmospheric pressure of that liquid is lower.
How does boiling point work?
A liquid ‘s boiling point is the temperature at which its vapor pressure is equal to that of the gas above it. The normal boiling point of a liquid is the temperature at which one atmosphere (760 torr) is equal to its vapor pressure. Microscopic view of the boiling water inside a bubble.
What affects boiling point of water?
The surrounding pressures are the greatest determinant of the boiling point of a liquid. The air pressure in an open system is most definitely the atmosphere on earth. For instance, water reaches the standard atmospheric pressure at 100 degrees centigrade. Water can boil at a lower temperature as elevation increases.
To learn more about melting point and boiling point, download BYJU’S – The Learning App.

Comments