Table of Contents
What is Tin (II) chloride?
SnCl2 in its solid form is a crystalline mass with the chemical name Tin (II) chloride.
Tin (II) chloride is also called Tin dichloride or Dichlorotin or Tin Protochloride, or Stannous chloride. It has a lone pair of electrons where the molecule in the gaseous phase is bent.
It appears as a white crystalline solid which is odourless. It is toxic when swallowed and irritates eyes and skin when comes in contact. It is widely used in the manufacturing of pharmaceuticals, as a tanning agent, and in the production of dyes.
Properties of Tin (II) chloride – SnCl2
| SnCl2 | Tin (II) chloride |
| Molecular weight of SnCl2 | 189.60 g/mol (anhydrous) |
| Density of Tin (II) chloride | 3.95 g/cm3 (anhydrous) |
| Boiling point of Tin (II) chloride | 623 °C |
| Melting point of Tin (II) chloride | 247 °C |
Tin (II) chloride Structure – SnCl2
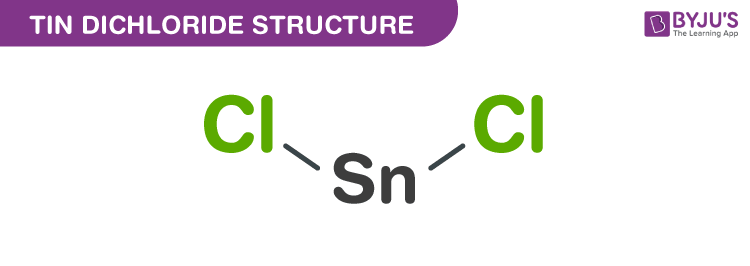
Tin (II) chloride structure – SnCl2
The exact mass and the monoisotopic mass of Tin dichloride is 189.84 g/mol. The number of hydrogen bond acceptors and the number of hydrogen bond donors equals to zero. This compound is canonicalized and has one covalently bonded unit only.
SnCl2 Uses (Tin (II) chloride)
-
-
-
- Tin (II) chloride is used as a strong reducing agent.
- Used in the manufacturing of pharmaceutical products.
- Used in removing ink stains.
- Used as an additive in lubricating oils.
- Used as a catalyst.
- Used to manufacture colour pigments.
- Used in tin-plating of steel.
- Used in radionuclide angiography.
- Used as a mordant in textile dyeing.
- Used to produce plastic polylactic acid.
-
-
Production of Tin (II) chloride
Anhydrous Dichlorotin is obtained by treating dry hydrogen chloride gas with tin metal. The dihydrate is prepaid by using hydrochloric acid (HCl):
Sn (s) + 2 HCl (aq) → SnCl2 (aq) + H2 (g)
The dihydrate is dehydrated to anhydrous by treating with acetic anhydride.
Health Hazards
Tin Protochloride is toxic and corrosive but non-combustible compound. Inhaling, swallowing or skin contact with this compound can cause severe injuries or lead to death. In its molten form, it may result in severe burns on the skin and in eyes. When heated, it liberates corrosive, irritating, and toxic gases.
Frequently Asked Questions
What is tin chloride made of?
For tin-plating pipes, a solution of tin(II) chloride containing a small amount of hydrochloric acid is used to produce tin-cans. An electric potential is applied, and through electrolysis, tin metal is formed at the cathode.
Is Tin (II) chloride ionic or covalent?
In SnCl2, Sn has +2 oxidation state. According to Fajan’s rule, the central metal with more oxidation number will be considered to be more covalent. Therefore, SnCl4 is more covalent than SnCl2.
Does tin dissolve in hydrochloric acid?
Metallic tin is brittle and abrasive. It dissolves gradually in dilute non oxidizing acids, or in hot concentrated HCl more readily. It interacts with HNO3 to form the meta stannic acid, H2SnO3, an insoluble white material in alkali or acids.
Is tin a metal?
Tin (Sn), a chemical element belonging to the carbon family, in the periodic table group 14 (IVA). It is a smooth, silvery white metal with a bluish tinge, an alloy of copper common to the ancients in bronze.
Is SnCl4 aqueous?
Anhydrous Stannic chloride is a colourless fuming liquid with a pungent scent. With heat evolution it is soluble in cold water and decomposed by hot water to form hydrochloric acid. Tin Chloride (SnCl4) is a food-borne antioxidant.

Comments