What is Maltose?
Maltose is formed from two molecules of glucose. When the two molecules bond together, a molecule of water (H2O) is removed. Maltose, malt sugar, C12H22O11.H2O, is prepared from starch by diastase. Hydrolysis by acids or maltose gives only d-glucose. In lean dough no added sugar, most of the sugar available to yeast is maltose, derived from starch. Maltose behaves like an early product of photosynthesis, rather than a storage product such as starch and its degradation products.
Other names – Cextromaltose, Maltobiose, Maltodiose, D-Maltose
| C12H22O11 | Maltose |
| Density | 1.54 g/cm³ |
| Molecular Weight/ Molar Mass | 342.3 g/mol |
| Specific Rotation | +130.5 [α]20D |
| Melting Point | 160 to 165 °C |
| Chemical Formula | C12H22O11 |
Table of Contents
- Maltose Structure – C12H22O11
- Physical Properties of Maltose – C12H22O11
- Chemical Properties of Maltose – C12H22O11
- Uses of Maltose – C12H22O11
- Frequently Asked Questions
Maltose Structure – C12H22O11
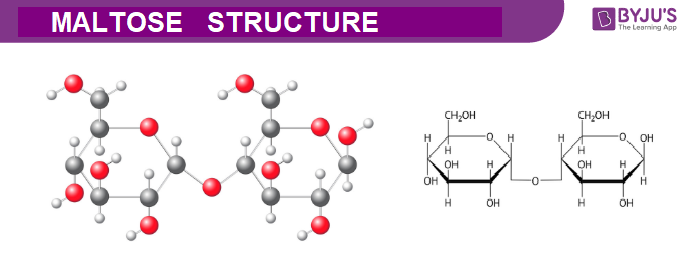
Physical Properties of Maltose – C12H22O11
| Odour | No odour |
| Appearance | White powder or crystals |
| Covalently-Bonded Unit | 1 |
| Heat capacity | 298.15 K |
| Complexity | 382 |
| Solubility | Soluble in water |
Chemical Properties of Maltose – C12H22O11
-
- Maltose undergoes hydrolysis results in the formation of ethanol and carbon dioxide. The chemical equation is given below.
C12H22O11 + H2O → 4C2H5OH + 4CO2
-
- Maltose reacts with sulphuric acid form carbon dioxide, water and sulphur dioxide. The chemical equation is given below.
C12H22O11 + 24H2SO4 → 12CO2 + 35H2O + 24SO2
Uses of Maltose – C12H22O11
- Used for brewing as their composition is similar to wort. Also in doughs with strong fermenting yeasts as these are able to metabolize maltose quickly.
- Used as a carrying material for flavouring materials and volatile aromas. It is also used in infant foods.
- The medicinal use of maltose is based on its repair and protection of the liver, for which modern glucose is used.
Frequently Asked Questions
What are the uses of maltose?
Maltose is a disaccharide consisting of two units of glucose. It has a slightly sweet taste but digestion is its most important function. Since most carbohydrates are in a non-absorbable shape, it is essential that these carbohydrates are broken into smaller parts.
Is maltose a reducing sugar?
Similar to glucose, maltose is a reducing sugar. Since the ring of one of the two glucose units will open to present a free group of aldehydes, the other is unable because of the glycosidic bond. The maltose enzyme, which catalyses the hydrolysis of the glycosidic bond, can break it down to glucose.
What are the sources of maltose?
Maltose is a sugar that is a component of malt, which is a substance that is obtained by allowing the grain to soften and germinate in water. In partially hydrolysed starch products such as maltodextrin, corn syrup, and acid-thinned starch, it is also present in highly variable amounts.
Recommended Videos
Classification Of Carbohydrates And Its Structure


Comments