What is Crystal Structure?
A crystal structure is made of atoms. A crystal lattice is made of points. A crystal system is a set of axes. In other words, the structure is an ordered array of atoms, ions or molecules.
Crystal Structure is obtained by attaching atoms, groups of atoms or molecules. This structure occurs from the intrinsic nature of the constituent particles to produce symmetric patterns. A small group of a repeating pattern of the atomic structure is known as the unit cell of the structure. A unit cell is the building block of the crystal structure and it also explains in detail the entire crystal structure and symmetry with the atom positions along with its principal axes. The length, edges of principal axes and the angle between the unit cells are called lattice constants or lattice parameters.
Table of Contents
Recommended Videos

Unit Cell
Crystals use x-rays, which excite signals from the atom. The signals given by these atoms have different strengths, and they usually depend upon the electron density distribution in closed shells. The signals released by the atoms varies. Lighter the atoms, weaker is their signals. The mutual arrangement of atoms is also known as crystal structures. Crystal structures are derived from the physical density and chemical formulas of solids.
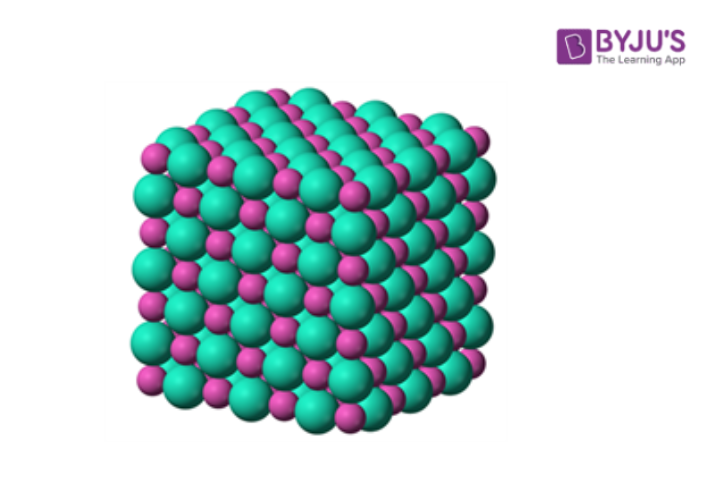
Unit cell can be defined as the smallest part of a component of the crystal. A group of atoms, ions or molecules, which are arranged together in a pure manner to build the crystal. The unit cells are structured in three-dimensional space, which describes the bulk arrangement of atoms of the crystal.
Crystal Systems
A Crystal System refers to one of the many classes of crystals, space groups, and lattices. In crystallography terms, lattice system and crystal, the system are associated with each other with a slight difference. Based on their point groups crystals and space groups are divided into seven crystal systems.
The Seven Crystal Systems is an approach for classification depending upon their lattice and atomic structure. The atomic lattice is a series of atoms that are organized in a symmetrical pattern. With the help of the lattice, it is possible to determine the appearance and physical properties of the stone. It is possible to identify which crystal system they belong to. In a Cubic System crystals are said to represent the element earth. They are Seven Crystal Systems and are stated below with illustrated examples.
The Seven Crystal Systems
-
-
-
-
Triclinic System:
It is the most unsymmetrical crystal system. All three axes are inclined towards each other, and they are of the same length. Based on the three inclined angles the various forms of crystals are in the paired faces. Some standard Triclinic Systems include Labradorite, Amazonite, Kyanite, Rhodonite, Aventurine Feldspar, and Turquoise.

-
Monoclinic System:
It comprises three axes where two are at right angles to each other, and the third axis is inclined. All three axes are of different length. Based on the inner structure the monoclinic system includes Basal pinacoids and prisms with inclined end faces. Some examples include Diopside, Petalite, Kunzite, Gypsum, Hiddenite, Howlite, Vivianite and more.

-
Orthorhombic System:
It comprises three axes and is at right angles to each other. There are different lengths. Based on their Rhombic structure the orthorhombic system includes various crystal shapes namely pyramids, double pyramids, rhombic pyramids, and pinacoids. Some common orthorhombic crystals include Topaz, Tanzanite, Iolite, Zoisite, Danburite and more.

-
Trigonal System:
Angles and axis in a trigonal system are similar to Hexagonal Systems. At the base of a hexagonal system (ross-section of a prism), there will be six sides. In the trigonal system (base cross-section) there will be three sides. Crystal shapes in a trigonal system include three-sided pyramids, Scalenohedral and Rhombohedra. Some typical examples include Ruby, Quartz, Calcite, Agate, Jasper, Tiger’s Eyes and more.

-
Hexagonal System:
It comprises four axes. The three a1, a2 and a3 axes are all contained within a single plane (called the basal plane) and are at 120°. They intersect each other at an angle of sixty degrees. The fourth axis intersects other axes at right angles. Crystal shapes of hexagonal systems include Double Pyramids, Double-Sided Pyramids, and Four-Sided Pyramids. Example: Beryl, Cancrinite, Apatite, Sugilite, etc.

-
Tetragonal Systems:
It consists of three axes. The main axis varies in length; it can either be short or long. The two-axis lie in the same plane and are of the same length. Based on the rectangular inner structure the shapes of crystal in tetragonal include double and eight-sided pyramids, four-sided prism, trapezohedrons, and pyrite.

-
Cubic System:
Cubic system is the most symmetrical one out of the seven crystal system. All three angles intersect at right angles and are of equal length. Crystal shapes of a cubic system based on inner structure (square) include octahedron, cube, and Hexaciscoherdron. Example: Silver, Garnet, Gold, and Diamond.

-
-
-
Frequently Asked Questions – FAQs
Why is crystal structure important?
The distinction between two minerals: graphite and diamond, is a perfect example of the value of crystal structure. This tells us that not only is it important to know what elements are in the mineral, but how those elements are stacked together is also very important to know.
Do rocks have crystal structure?
The composition of the crystal is based on the conditions in which the mineral forms. The minerals with the same chemical formula but different crystal shapes are polymorphs. Conditions such as temperature (T) and pressure ( P) are the conditions, since they influence ionic radii.
What is the difference between crystals minerals and rocks?
A mineral is an inorganic substance or compound that occurs naturally and has an ordered internal structure and chemical composition, crystal shape, and physical properties that are distinctive. A rock, or a mass of undifferentiated mineral matter, is an accumulation of one or more minerals.
What determine the shape of a crystal?
A number of variables, such as the size and length of their surfaces (known as ‘faces’) and sides, and the angles between them, define the shapes of crystals. These forms are named for their geometry – for example, the ‘cubic or isometric’ crystal group belongs to crystals centred on cubes.
To know more about the Atomic Structure, Crystal structure, and their classification, register with BYJU’S.


Comments