Carbohydrate is a group of organic compounds occurring in living tissues and foods in the form of starch, cellulose, and sugars. The ratio of oxygen and hydrogen in carbohydrates is the same as in water i.e. 2:1. It typically breaks down in the animal body to release energy.
What are Carbohydrates? (Carbohydrate Definition)
Cn(H2O)n is the generic formula for all carbohydrates. This formula is only valid for simple sugars, which are made up of the same amount of carbon and water.
Originally the term carbohydrate was used to describe compounds that were literally “carbohydrates,” because they had the empirical formula CH2O. Carbohydrates have been classified in recent years on the basis of carbohydrate structures, not their formulae. Such aldehydes and ketones are now known as polyhydroxy. Cellulose, starch, and glycogen are among the compounds that belong to this family.
What is the General Formula of Carbohydrates?
The general formula for carbohydrates is Cx(H2O)y.
Carbohydrates (or sugars) were originally believed to be “hydrates of carbon,” because they have the general formula Cx(H2O)y.
Definition of Carbohydrates in Chemistry
Chemically, carbohydrates are defined as “optically active polyhydroxy aldehydes or ketones or the compounds which produce units of such type on hydrolysis”.
The substance most people refer to as “sugar” is the sucrose disaccharide, which is extracted either from sugar cane or beets. Sucrose is the disaccharide most sweet. It’s approximately three times sweet as maltose, and six times sweet as lactose.
In recent years, in many consumer products, sucrose has been replaced with corn syrup, which is obtained when the polysaccharides in cornstarch are broken down. Corn syrup is primarily glucose, which is as sweet as sucrose only about 70 per cent.
Related Topics |
Carbohydrates are also called saccharides which is a Greek word it means sugar because almost all carbohydrates have a sweet taste.
Recommended Videos
Classification of Carbohydrates

Introduction to Carbohydrates

Carbohydrates Definition in Science
The term carbohydrate or hydrates of carbon is derived from its basic elemental formula in which carbon is joined to hydrogen and oxygen present in the same ratio as in water. Chemically carbohydrates are polyhydroxy aldehydes or ketones, their simple derivatives or their polymers.
Carbohydrates in grains are classified based on their chemical structures or their digestibility when consumed by humans as food or by livestock as feed. Simple carbohydrates which are sweet and soluble in water are also known as sugars or disaccharides and the ending of the names of most sugars is -ose. Thus, we have such names as sucrose for ordinary table sugar, glucose for principal sugar in blood and maltose for malt sugar.
Carbohydrates Structure
Historically carbohydrates were defined as substances with the empirical formula Cn(H2O)m. The common sugars such as glucose and fructose or sucrose fit this formula, but nowadays the convention is to regard as a carbohydrate a polyhydroxy aldehydes or polyhydroxy ketone with the classical formula, a molecule closely related to it, or oligomers or polymers of such molecules. Their study evolved as a separate sub-discipline within organic chemistry for practical reasons – they are water-soluble and difficult to crystallise so their manipulation demanded different sets of skills from classical “natural products” such as terpenes, steroids, alkaloids, etc.
The term “monosaccharide” refers to a carbohydrate derivative possessing a single carbon chain; “disaccharide” and “trisaccharide” refer to molecules containing two or three such monosaccharide units joined together by acetal or ketal linkages. “Oligosaccharide” and “polysaccharide” refer to larger such aggregates, with “a few” and many monosaccharide units, respectively. Current usage seems to draw the distinction between “few” and many at around 10 units.
By the middle of the nineteenth century, a number of relatively pure carbohydrates such as sucrose, cellulose from cotton, starch, glucose, fructose, mannose and lactose were known to the chemists of Europe, especially in Germany. In 1878, Emil Fischer synthesized phenylhydrazine for his thesis at the University of Munich. In 1884 he further discovered that carbohydrates gave crystalline phenylosazone in which two phenyl hydrazines reacted with the aldehyde group and the carbon adjacent to the aldehyde group.
Carbohydrates Formula
Carbohydrates are large macromolecules consisting of carbon (C), hydrogen (H) and oxygen (O) and have the general Cx(H2O)y formula. Carbohydrates have the general formula Cx(H2O)y. The hydrate of carbon is known as carbohydrates. They contain hydrogen and oxygen in the same proportion as in water. It may be noted that there are some carbohydrates which do not conform to the formula Cx(H2O)y, for example, 2-deoxyribose C5H10O4. However, most of them conform to the formula Cx(H2O)y.
Carbohydrates are also called sugars in general some partially methylated sugars and amino sugars and amino sugars naturally and one natural nitro sugar is known. All carbohydrates are polyhydroxy aldehydes or ketones or substances that yield these on hydrolysis.
Haworth projections represent the cyclic structures of monosaccharides. Monosaccharides contain either an aldehyde group (aldose) or a ketone group (ketose) and several -OH groups. Straight chain forms of sugars cyclize in solution to form ring structures containing an ether linkage. Glycosidic bonds form between monosaccharides forming disaccharides and polysaccharides. Carbohydrates are used as energy sources and energy reserves.
Sources of Carbohydrates
We know carbohydrates are an important part of any human’s diet. Some common sources of carbohydrates are:
- Potatoes
- Maze
- Milk
- Popcorn
- Bread
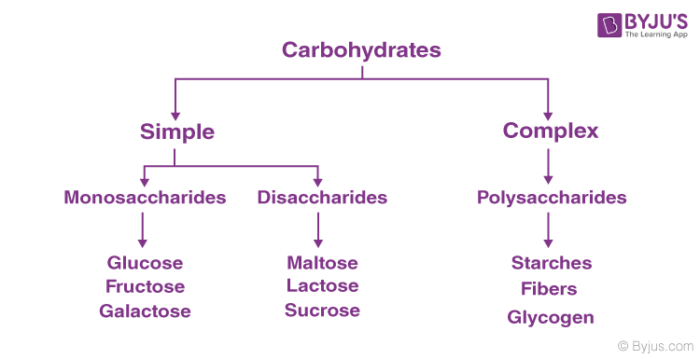
Types of Carbohydrates
Types of Carbohydrates – Simple Carbohydrates
Simple carbohydrates are the basic type of carbs. Soft drinks, candy, cookies and other sweet snacks contain simple carbohydrates. These foods are often made with white sugar, a form of processed sugar.
Simple carbohydrates also are found in natural sugars. Fruit, milk and vegetables contain natural sugars. Honey is a natural sugar as well. People eat natural sugar in its original form. Simple carbohydrates are easier to handle because they are less (or simpler) complex. They come from fruit and sugar stuff, as well as pretty much anything else that’s sweet. The human body can rapidly break down these things, and that is where some of the problems lie.
There is only one sugar unit in the monosaccharides, so they are the smallest of the carbohydrates. “The small size of monosaccharides gives them a special role in digestion and metabolism. (The prefix” mono- “means” one.) Before they can be ingested into the gastrointestinal tract, food carbohydrates have to be broken down into monosaccharides and they also flow in monosaccharide form in the blood.
Types of Carbohydrates – Complex Carbohydrates
Complex carbohydrates represent an important energy source for your body. They provide the sustained fuel your body needs for exercise, daily living activities and even rest.
Complex carbohydrates are often single units (monosaccharides), which are bound together. The oligosaccharides contain two to ten simple units of sugar. Polysaccharides contain hundreds and thousands of monosaccharides which are related. Complex carbohydrates have fairly long-lasting energy.
The different types of carbohydrates can be classified on the basis of their behaviour in hydrolysis. They are mainly classified into three groups:
- Monosaccharides
- Disaccharides
- Polysaccharides
1. Monosaccharides
Monosaccharide carbohydrates are those carbohydrates that cannot be hydrolyzed further to give simpler units of polyhydroxy aldehyde or ketone. If a monosaccharide contains an aldehyde group then it is called aldose and on the other hand, if it contains a keto group then it is called a ketose.
Structure of Carbohydrates – Glucose
One of the most important monosaccharides is glucose. The two commonly used methods for the preparation of glucose are
- From Sucrose: If sucrose is boiled with dilute acid in an alcoholic solution then we obtain glucose and fructose.
- From Starch: We can obtain glucose by hydrolysis of starch and by boiling it with dilute H2SO4 at 393K under elevated pressure.
Glucose is also called aldohexose and dextrose and is abundant on earth.
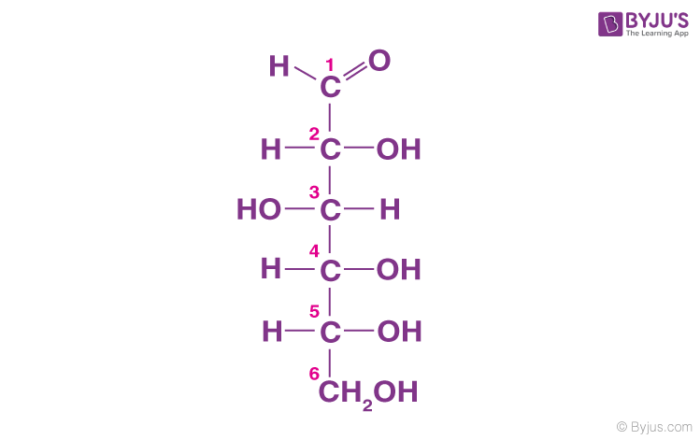
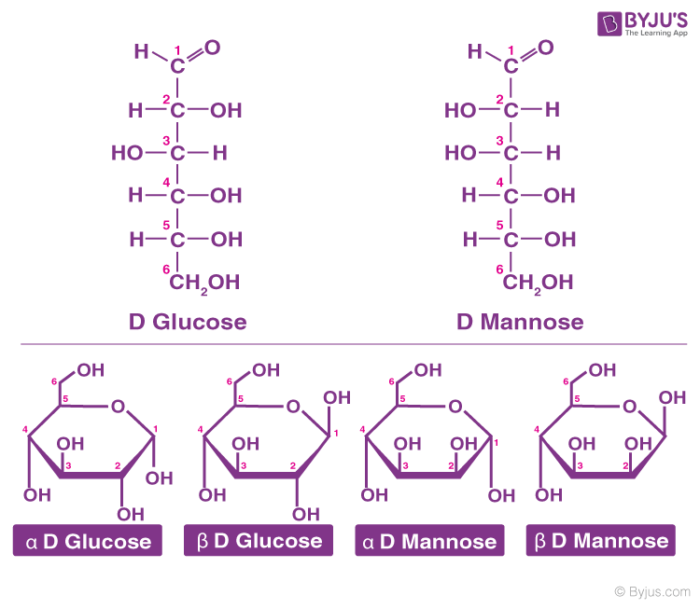
- Glucose is named as D (+)-glucose, D represents the configuration whereas (+) represents the dextrorotatory nature of the molecule.
- The ring structure of glucose can explain many properties of glucose which cannot be figured by open-chain structure.
- The two cyclic structures differ in the configuration of the hydroxyl group at C1 called anomeric carbon. Such isomers i.e. α and β form are known as anomers.
- The cyclic structure is also called pyranose structure due to its analogy with pyran.
The cyclic structure of glucose is given below:
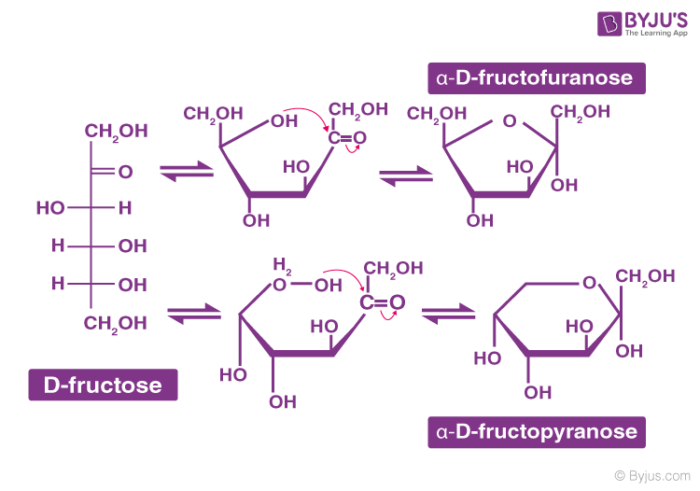
Structure of Carbohydrates – Fructose
It is an important ketohexose. The molecular formula of fructose is C6H12O6 and contains a ketonic functional group at carbon number 2 and has six carbon atoms in a straight chain. The ring member of fructose is in analogy to the compound Furan and is named furanose. The cyclic structure of fructose is shown below:
Examples of Carbohydrates
Here are a few examples of where you’ll find the most carbs:
- Dairy Products – Yogurt, Milk, Ice cream
- Fruits – Fruit juice or Whole fruit
- Grains – Cereal, Bread, Wheat, Rice
- Legumes – Plant-based proteins, Beans
- Starchy Vegetables – Corn, Potatoes
2. Disaccharides
- On hydrolysis, disaccharides yield two molecules of either the same or different monosaccharides.
- The two monosaccharide units are joined by oxide linkage which is formed by the loss of water molecule and this linkage is called glycosidic linkage.
- Sucrose is one of the most common disaccharides which on hydrolysis gives glucose and fructose.
- Maltose and Lactose (also known as milk sugar) are the other two important disaccharides.
- In maltose, there are two α-D-glucose and in lactose, there are two β-D-glucose which are connected by an oxide bond.
Also, Check ⇒ Structure & Properties of Maltose
3. Polysaccharides
- Polysaccharides contain long monosaccharide units joined together by glycosidic linkage.
- Most of them act as food storage for e.g. Starch. Starch is the main storage polysaccharide for plants.
- It is a polymer of α glucose and consists of two components-Amylose and Amylopectin.
- Cellulose is also one of the polysaccharides that are mostly found in plants.
- It is composed of β-D- glucose units joined by a glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
Frequently Asked Questions – FAQs
What are carbohydrates?
Carbohydrates are the sugars, starches and fibres present in the products of fruits, grains, vegetables and milk. The American Diabetes Association states that carbohydrates are the primary source of energy for the body. They are called carbohydrates, as they contain carbon, hydrogen and oxygen at the chemical level.
What types of foods are carbohydrates?
Carbohydrates are present in a wide range of safe as well as unhealthy foods — bread, beans, milk, popcorn, potatoes, cookies, pasta, soft drinks, corn, and cherry pie. They come in a range of shapes too. The most natural and abundant types are sugars, starches and fibres.
What are the major functions of carbohydrates?
The four primary carbohydrate functions in the body are to provide energy, store energy, create macromolecules and spare protein and fat for other uses. Glucose energy is processed in the form of glycogen, with most in the muscle and liver.
What are the main carbohydrates?
Foods rich in carbohydrates include bread, vegetables and fruits, as well as dairy. Carbohydrates are the sugars, starches and fibres present in the products of fruits, grains, vegetables and milk. Even though often maligned in trendy diets, carbohydrates are essential to a healthy diet as one of the basic food groups.
What are the two sources of carbohydrates?
Healthy carbohydrate sources include both animal and plant food sources, such as fresh fruits, tomatoes, corn, potatoes, meat, and milk products. Examples that are not safe include soda, white bread, added sugar, pastries and other highly processed food.
What is a simple carbohydrate?
The body rapidly breaks down simple carbohydrates to be used as energy. Simple carbohydrates are naturally found in foods such as fruit, milk, and dairy products. In processed and refined sugars such as candy, table sugar, syrups and soft drinks, are also found.
What is a complex carbohydrate?
Simple carbohydrates consist of sugar molecules, which are bound together in long, complex chains. Foods such as peas, beans, whole grains, and vegetables contain complex carbohydrates. Within the body, both simple and complex carbohydrates are converted into glucose ( blood sugar) and used as energy.
What is the difference between complex and simple carbohydrates?
Simple carbohydrates are present in such foods as table sugar and syrups. Complex carbohydrates contain longer sugar molecular chains than mere carbohydrates. Since complex carbohydrates have longer chains, they take longer than simple carbohydrates to break down and provide more lasting energy in the body.
Also, Read ⇒ Properties of Amylose
Glycogen: These carbohydrates are stored mainly in the animal body. It is present in the liver, muscles, and brain. When the body needs glucose, enzymes break the glycogen.


Comments