What is Cellulose?
Cellulose is the most abundant organic compound on earth with a chemical formula (C6H10O5)n.
Cellulose is a complex carbohydrate consisting of oxygen, carbon, and hydrogen. It is chiral, tasteless and has no odour. A French chemist by name Anselme Payen was the first to discover cellulose in the year 1838. This organic compound is water-soluble and biodegradable.
In the year 1890, it was used to produce the first thermoplastic called celluloid. The amount of cellulose present in cotton is 90%. The amount of cellulose present in the wood is 40-45% and dried hemp is 57% respectively.
Properties of Cellulose – (C6H10O5)n
Many Properties of cellulose depend upon the degree of polymerization or chain length and the number of glucose molecules constituting the polymer molecule. Cellulose is odourless and insoluble in water and most organic solvents. It is biodegradable and chiral.
| (C6H10O5)n | Cellulose |
| Molecular Weight/ Molar Mass | 162.1406 g/mol |
| Density | 1.5 g/cm³ |
| Appears | White powder |
| Melting Point | 260–270 °C |
Structure of Cellulose– (C6H10O5)n
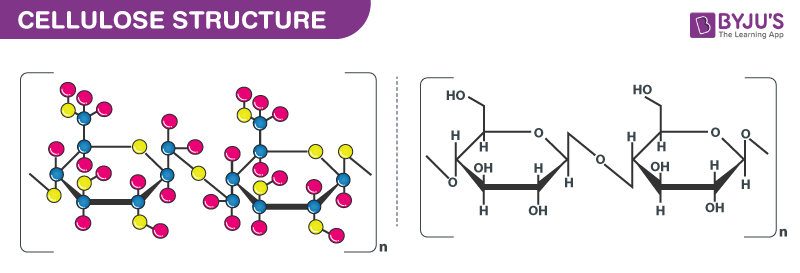
Structure of Cellulose– (C6H10O5)n
At high temperatures, It can be broken down into glucose by treating it with concentrated mineral acids. It is more crystalline when compared to starch. But starch goes from crystalline to amorphous transition at 60-70 degrees but cellulose, on the other hand, requires 320 degrees and a pressure of 25 megapascals.
Uses of Cellulose (C6H10O5)n
- It is used in the diet as a fibre supplement
- It is used to produce paperboard and paper products
- It helps as an additive in various food items
- It is used in the production of rayon
- It is used as a preservative in cheese as it plays the role of an anti-clumping agent
- It is used in making explosives
- It is used in the manufacturing of nitrocellulose
Recommended Video
Natural Polymers: Characteristics and Examples

Frequently Asked Questions – FAQs
What is cellulose used for?
Cellulose is used mainly in paperboard and paper production. Smaller amounts are converted into a wide variety of derivatives, such as cellophane and rayon. The conversion of cellulose from energy crops into biofuels such as cellulosic ethanol as a renewable fuel source is in progress.
Is cellulose a protein?
A protein is a polypeptide. Example- Polysaccharide: any of a class of carbohydrates with chains of monosaccharide molecules in its molecules. Examples include cellulose, glycogen, starch, etc.
Is cellulose a sugar?
Cellulose is a long chain of sugar molecules linked together which gives wood its remarkable strength. It is the key component of cell walls for plants and the essential building block for many textiles and papers. The cellulose chain links are one sugar type: ß-D-glucose.
Which foods contain cellulose?
Cellulose is an insoluble dietary fibre consisting of glucose polymers present in all cell walls of plants. Types of cellulose-containing foods include leafy green vegetables, such as kale, sprouts from Brussels and green peas.
Is cellulose branched or unbranched?
Cellulose is the main polysaccharide used for structural function in plants. This is one of the most common organic compounds found on the planet, obviously. Cellulose is an unbranched glucose residue polymer put together via beta-1,4 connections, which enables the molecule to form long, straight chains.
Learn more about the structure and properties of (C6H10O5)n from the expert faculties at BYJU’S.

Is (C6H10O5)n home compostable?
Since cellulose is easily digested by microbes, it can be added to compost bins.
What does ‘n’ mean in (C6H10O6)n?
Since cellulose is a polymer, ‘n’ denotes the degree of polymerization or the total number of repeating polymer units.