What is Acid Rain?
Acid Rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur react with rainwater and come down with the rain, then this results in Acid Rain.
Table of contents
- Definition Of Acid Rain
- Recommended Videos Of Acid Rain
- Causes Of Acid Rain
- Effect Of Acid Rain
- Real-Life Examples Acid Rain
- Prevention Of Acid Rain
- FAQs
Acid Rain Definition
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. It is often called acid rain as this concept contains many types of acidic precipitation.
The acidic deposition takes place in two ways: wet and dry. Wet deposition is any form of precipitation which removes acids from the atmosphere and places them on the surface of the earth. In the absence of precipitation, dry deposition of polluting particles and gases sticks to the ground through dust and smoke.
Recommended Videos Of Acid Rain

Causes of Acid Rain
The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rain. Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions that are given out from industries or by natural causes like lightning strike in the atmosphere releasing nitrogen oxides and volcanic eruptions releasing sulphur oxide.
According to the Royal Society of Chemistry, which considers him the “father of acid rain,” the word acid rain was invented in 1852 by Scottish chemist Robert Angus Smith. Smith decided on the word while studying rainwater chemistry near industrial towns in England and Scotland.
The regular clean rain we experience, even though it is not clean i.e water and carbon dioxide react together to form weak carbonic acid which essentially by itself is not extremely harmful. The reaction occurring is :
H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
The pH value of regular rainwater is around 5.7, giving it an acidic nature. The oxides of nitrogen and sulphur are blown away by the wind along with the dust particles. They settle on the earth’s surface after coming down in the form of precipitation. Acid rain is essentially a by-product of human activities which emit oxides of nitrogen and sulphur in the atmosphere. Example – the burning of fossil fuels, unethical waste emission disposal techniques.
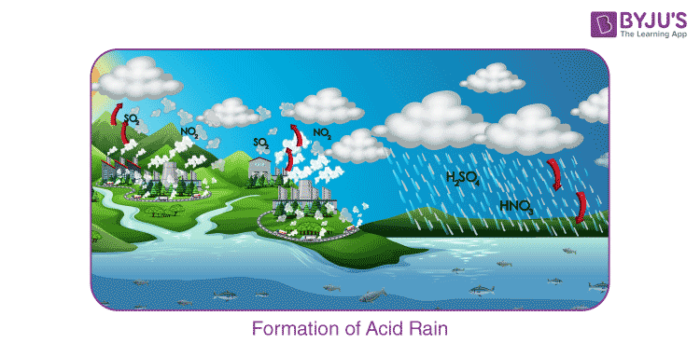
Sulphur dioxide and nitrogen dioxide undergo oxidation, and then they react with water resulting in the formation of sulphuric acid and nitric acid, respectively. The following reaction will clarify the acid formation reaction:
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
4NO2 (g) + O2 (g) + 2H2O (l) → 4HNO3 (aq)
Effects of Acid Rain
- Acid rain is very harmful to agriculture, plants, and animals. It washes away all nutrients which are required for the growth and survival of plants. Acid rain affects agriculture by the way it alters the composition of the soil.

- It causes respiratory issues in animals and humans.
- When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. It alters the chemical composition of the water, to a form which is actually harmful to the aquatic ecosystem to survive and causes water pollution.
- Acid rain also causes the corrosion of water pipes, which further results in leaching of heavy metals such as iron, lead and copper into drinking water.
- It damages the buildings and monuments made up of stones and metals.
Real-Life Examples
- Taj Mahal, one of the 7 wonders of the world, is largely affected by acid rain. The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as a domestic fuel, adding to this problem. Acid rain has the following reaction with the marble (calcium carbonate):
CaCO3(s) + H2SO4(l) → CaSO4(s) + H2O(l) + CO2(g)

The formation of calcium sulphate results in the corrosion of this beautiful monument.
- Statue of Liberty which is made of copper has also been damaged by the cumulative action of acid rain and oxidation for over 30 years and is, therefore, becoming green.
Prevention of Acid Rain
- The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur.
- Acid rain is harmful to animals, plants and the monuments.
- Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
Frequently Asked Questions – FAQs
What is acid rain and how is it caused?
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
What are the effects of acid rain?
The ecological consequences of acid rain are seen most strongly in marine habitats, such as streams, lakes and marshes where fish and other wildlife can be toxic. Acidic rainwater can leach aluminium from soil clay particles as it flows through the soil and then floods into streams and lakes.
What will happen if we don’t stop acid rain?
Sulphur dioxide and nitrogen oxide are the principal chemicals for acid rain. It can also influence humans since the acid goes into fruits, vegetables and animals. In other words, we can get really sick if acid rain doesn’t stop, and we eat those things. In general, acid rain affects men, but not directly.
What is acid rain? What are its harmful effects?
It has been shown that acid rain has detrimental effects on trees, freshwaters and soils, destroys insects and aquatic life-forms, causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and sculptures, as well as impacts on human health.
What are three ways to reduce acid rain?
Alternative energy sources should be used, such as solar and wind power. Renewable sources of energy are helping to reduce acid rain, as they produce much fewer emissions. There are other electricity sources as well, such as nuclear power, hydropower, and geothermal energy. Among these, the most extensive use is among nuclear and hydropower.
How does acid rain affect plants?
What is acid rain made of?
What is the primary source of acid rain?
Can acid rain damage buildings?
Can acid rain burn your skin?
Read more:




Nice informations
I love it
Really like the information tq
These notes are very helpful in my exams i scored 79/80 in science
these are very useful