What is Lactic Acid?
Lactic Acid is an organic acid with the chemical formula C3H6O3. It is also known as milk acid. When milk sugar (lactose) undergoes fermentation, the product obtained is lactic acid. It is found in cottage cheese, leban, sour milk, yoghurt, and Koumiss. Levo and Dextro are two forms of optical isomers of lactic acid.
In the year 1780, Carl Wilhelm Scheele a Swedish chemist isolated lactic acid from sour milk for the first time. This compound is water-soluble and white in its solid state whereas it is colourless in its liquid state. The soluble salt of lactic acid like calcium lactate can be used as a source of calcium. The pH of 1 mM of lactic acid is about 3.51.
Table of Contents
- Properties of Lactic Acid-C3H6O3
- Lactic Acid Structure-C3H6O3
- C3H6O3 Uses (Lactic Acid)
- Frequently Asked Questions on Jute Fibre
Properties of Lactic Acid – C3H6O3
| C3H6O3 | Lactic Acid |
| Molecular Weight/ Molar Mass | 90.08 g/mol |
| pH | 3.51 |
| Boiling Point | 122 °C |
| Melting Point | 53 °C |
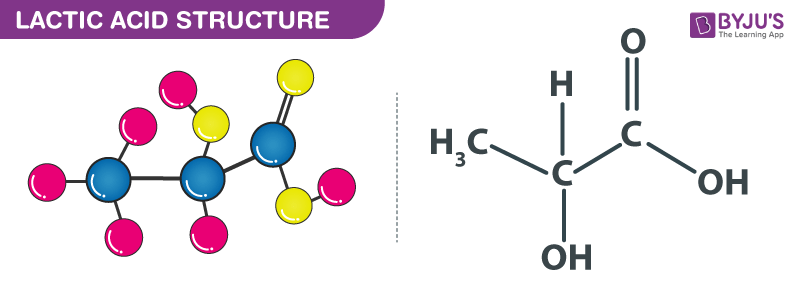
Lactic Acid structure – C3H6O3
C3H6O3 Uses (Lactic Acid)
- It is used in food and pharmaceuticals.
- It is used in making plastics.
- It helps in the coagulation of protein in milk.
- It is used in textile dyeing and leather tanning.
- Lactate plays an important role in the healing of traumatic brain injuries.
- The release of norepinephrine in the brain is signalled by lactate.
Frequently Asked Questions
What does lactic acid do to the body?
When the body is low in oxygen it requires the conversion of glucose into energy, which makes lactic acid. The build up of lactic acid can cause muscle pain, cramps and muscle fatigue. These symptoms are normal during physical exercise and are typically not a problem as the liver has the ability to break down any excess lactate.
Why does lactic acid build-up occur?
Lactic acid accumulation happens when the muscles don’t have enough oxygen to break down glucose and glycogen. This process is often referred to as anaerobic metabolism. Too much L-lactate is responsible for most types of lactic acidosis.
Which foods contain lactic acid?
Lactic acid is mainly present in sour dairy products, such as yoghurt, koumiss, kefir, laban, and some cottage cheese. The casein is coagulated (curdled) by lactic acid in fermented milk. Lactic acid also has the sour taste of sourdough bread.
Learn more about the structure and properties of C3H6O3 from the expert faculties at BYJU’S.


Comments