What are Organic Solvents?
Organic solvents are used by everyone in most of their daily activities starting right from the disinfectant treatment to the removal of tough grease stains. The perfumes or cologne we use, laundry detergents which are used to keep clothes fresh and clean, all these products contain those ingredients called as Organic solvents.
Table of Contents
- Types of Organic Solvents
- Properties of Organic Solvents
- Applications of Organic Solvents
- Frequently Asked Questions-FAQs

Organic solvents are those chemicals compounds having carbon-based molecular structure. These are widely used in dissolving material in-order to create a solution, or even in the extraction of one material from another material. In general, a solvent refers to the substance which is capable of dissolving any other substance. But, being carbon-based, all these solvents have carbon atoms in the structure of compounds. Consider the example of organic solvent benzene having six carbon atoms present in the organic solvent.
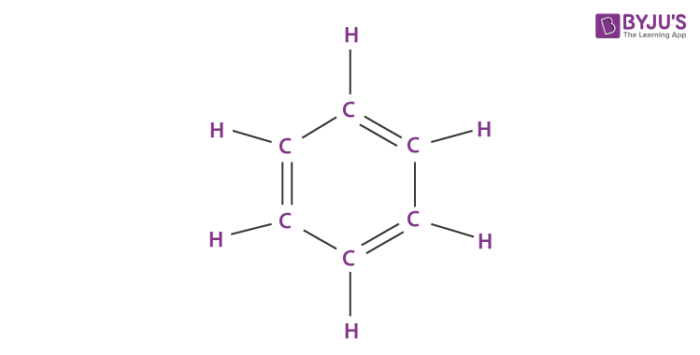
The molecular structure of an organic solvent always contains a carbon atom and some have hydrogen atoms. These solvents are mainly categorized based upon their molecular structures as natural and synthetic solvents.
Natural solvents – These are the solvents which are naturally produced by living organisms.
Synthetic Solvents – These are the solvents that are produced as a result of chemical reactions occurring in various organic compounds.
Types of Organic Solvents
Based upon the structure and the functional group, the different types of organic solvents are as explained below-
- Aliphatics solvents– These solvents belong to the class of alkenes. They are said to be nonpolar in nature. Some applications of such solvents include oil extraction, paint, dye, pharmaceuticals, polymerization, and adhesives.
- Aromatic solvents– These like the aliphatic are said to be nonpolar solvents. They are utilized as industrial solvents for adhesives, paints, printing inks, extraction processes, decreasing, in insecticides, etc.
- Carbonyls solvents– These include esters. They are said to exhibit polar properties and are used in the nail paint removers, electronic cleaners, circuit boards, decaffeination, in glues, and also in food flavouring substances.
Some of the other solvents include alcohols which are used in various industrial and commercial applications.
Properties of Organic Solvents
Organic solvents do exhibit various physical and chemical properties as given below-
- Organic solvents are volatile in nature– Volatile solvents are those which have the ability to vaporize. Organic solvents possess these properties. Due to the nature of volatility, organic solvents release smell when released into the air.
- Organic solvents exhibit a low boiling point- Organic solvents are said to have very low boiling points. Due to this low boiling point, they are highly volatile.
- Organic solvents are colourless liquids- These are clear liquids and have lower molecular weights.
Applications of Organic Solvents
Organic solvents find its uses in different areas. These are used in coatings, polishes, as a paint thinner and remover(toluene), as cleaning agents, as a nail polish remover(acetone, ethyl acetate, methyl acetate), as an industrial as well as consumer degreasers, detergents, perfumes, spot removers and also in various chemical syntheses and processes.
Frequently Asked Questions – FAQs
What is the strongest solvent?
The “universal solvent” is called water because it is able to dissolve more liquids than any other liquid. For any living being on earth, this is important.
Is acetone Protic or aprotic?
However, despite the fact that acetone is moderately acidic, and not substantially less acidic than alcohols. Then again, owing to their comparatively high acidity, acetone (and other carbonyl containing solvents) are also weak solvents when utilizing solid bases.
What are fat solvents?
Solvents of fat. Chemical liquids that are noteworthy for their lipid-dissolving ability; typically, but not always, water-immiscible; e.g. diethyl ether, tetrachloride carbon. Synonym: solvents which are nonpolar.
Why is acetone a good solvent for fat?
Acetone contains both non-polar methyl groups as well as polar carbonyl groups, so it also has the ability to dissolve non-polar compounds. Fat (lipids) are non-polar in nature. That means that in acetone, fats dissolve.
Why is acetone a better solvent than water?
Owing to its ability to dissolve both polar and nonpolar compounds, acetone is a strong solvent, while other solvents can only dissolve one or the other. Secondly, since it is miscible, acetone is a strong solvent, ensuring it has the potential to blend in all amounts with water.

Comments