What is Sulfuric Acid?
Sulfuric acid (H2SO4) is a strong acid with hygroscopic and oxidizing properties. Sulfuric Acid is a mineral acid with the chemical formula H2SO4.
Sulfuric acid is also known as Mattling acid or Oil of vitriol. It has a strong acidic nature and is corrosive. At higher concentrations, it acts as an oxidizing agent and dehydrating agent. It is a syrupy liquid which is odourless and has no colour. It is water-soluble and releases heat when dissolved in water. It is widely used in the manufacturing of fertilizers. It is also used in chemical synthesis and wastewater processes.
Anhydrous sulfuric acid has a dielectric constant of around 100 and is a very polar liquid. It is perhaps the most important heavy industrial chemical, with large-scale uses in a wide range of industries.
Sulfuric acid is a very strong acid; in aqueous solutions, it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4−). Hydrogen sulfate ions further ionise in very dilute solutions to give sulphate ions(SO42-).
Table of Contents
Properties of Sulfuric Acid – H2SO4
| H2SO4 | Sulfuric Acid |
| Molecular Weight/ Molar Mass | 98.079 g/mol |
| Density | 1.84 g/cm³ |
| Boiling Point | 337 °C |
| Melting Point | 10 °C |
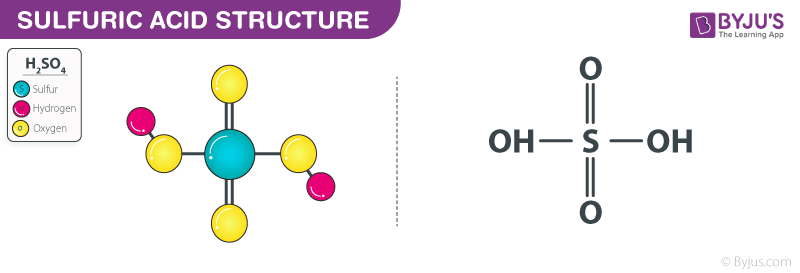
Sulfuric Acid Structure – H2SO4
H2SO4 Uses (Sulfuric Acid)
- It is used in making fertilizers
- It is used in the production of steel and iron
- It is used in chemical manufacturing industries
- It is used in petroleum refining
- It is used to produce phosphoric acid
- It used as a cleaning agent in industries to remove the rust from steel and iron
- It is used as a catalyst to convert cyclohexanone oxime to caprolactam used to make nylon
- It is used in lead-acid batteries as an electrolyte
- It is used in making ammonium sulfate
- It is used in storage batteries
FAQs
1. What is sulfuric acid used for?
The primary application of sulfuric acid is in fertilizer processing, e.g., lime superphosphate and ammonium sulfate. It is commonly used in chemical processing, e.g. in the manufacture of hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and medicines.
2. Which is the world’s strongest acid?
Carborane superacid can be considered the strongest solar acid in the world because fluoroantimonic acid is in reality a mixture of hydrofluoric acid and pentafluoride antimony.
3. How do you neutralize sulfuric acid?
Pour the baking soda directly into a spill of acid. This will neutralize light acids such as vinegar, or even toxic, strong acids such as muriatic and sulphuric acids. Douse with the baking soda (sodium bicarbonate, NaHCO3) in the entire contaminated region to neutralize the acid.
4. Can you neutralize sulfuric acid with water?
Sulphuric acid can not be neutralised by water but can be diluted with water. Add drops of acid to water carefully with constant stirring as the reaction is exothermic. To neutralise sulphuric acid, strong bases like NaOH can be used.
5. Is sulfuric acid ionic or molecular?
Water forms a compromise between H2O and H3O+ and -OH. That doesn’t make anything ionic. Sulfuric acid is a compound with covalence since all the bonds are covalent. But it ionises in solution to give hydronium and sulphate ions.
Also, Read:
| Benzoic Acid | Boric Acid |
| Sodium Hydroxide | Phosphoric Acid |
Register to know more about the importance of H2SO4 and its structure in depth from the expert faculties at BYJU’S.

Comments