What is Benzoic Acid?
Benzoic acid is an organic compound which is described by the chemical formula C6H5COOH. It consists of a carboxyl group attached to a benzene ring. Therefore, benzoic acid is said to be an aromatic carboxylic acid. This compound exists as a crystalline, colourless solid under normal conditions. The term ‘benzoate’ refers to the esters and salts of C6H5COOH.
The commercial production of benzoic acid is done via the partial oxidation of toluene with oxygen, catalyzed by manganese or cobalt naphthenates. This chemical reaction is illustrated below.
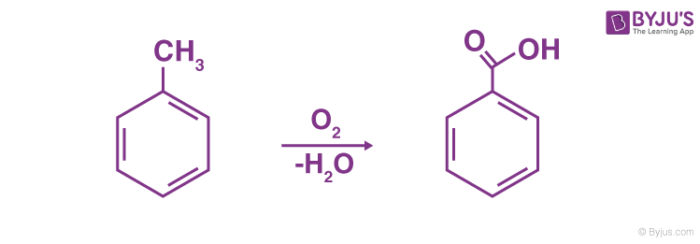
Another industrial method of preparing benzoic acid is by reacting tri-chlorotoluene with calcium hydroxide in the presence of water, and the treatment of the calcium benzoate product with hydrochloric acid.
Structure
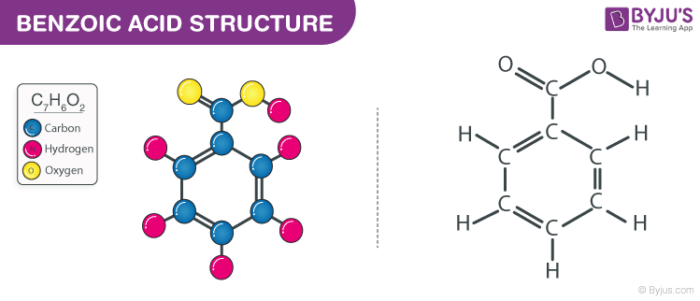
The structure of a C6H5COOH molecule is illustrated below. This molecule consists of a benzene ring to which a carboxyl functional group is linked. The molecule consists of 7 carbon atoms, 6 hydrogen atoms, and 2 oxygen atoms.
Properties of Benzoic Acid
The physical and chemical properties of C6H5COOH are discussed in this subsection.
Chemical Data
| Benzoic Acid Formula | C7H6O2 or C6H5COOH |
| Molecular Weight/ Molar Mass | 122.12 g/mol |
| Density | 1.27 g/cm3 at 15oC |
| Boiling Point | 523K |
| Melting Point | 395 K |
Physical Properties
- Benzoic acid has a colourless appearance in its solid state, which is of a crystalline nature.
- The crystal structure is monoclinic.
- The presence of the aromatic ring gives this compound a faintly pleasant odour.
- At a temperature of 130oC, the density of this compound reduces to 1.075 grams per cubic centimetre.
Chemical Properties
- It is soluble in water, and the solubility at 25oC and 100oC is 3.44 g/L and 56.31 g/L respectively.
- It is soluble in benzene, carbon tetrachloride, acetone, and alcohols.
- The acid dissociation constant (pKa) of benzoic acid corresponds to 4.2
- Its reactions can occur at the carboxyl group or even at the aromatic ring.
Uses of Benzoic Acid
Some important uses of C6H5COOH are listed below.
- The production of phenol involves the use of benzoic acid.
- This compound is used in ointments that prevent or treat fungal skin diseases.
- C6H5COOH is used as a preservative in the food industry.
- Benzoic acid is an ingredient in many cosmetic products, such as lipsticks.
- It is also a precursor to benzoyl chloride, which finds its application in making other chemicals, dyes, perfumes, herbicides and medicines.
- One of the components of toothpaste, mouthwash, and face wash creams is C6H5
- This compound is also used in the manufacture of dyes and in insect repellants.
Frequently Asked Questions
What are the uses of benzoic acid?
The primary use of benzoic acid is in the industrial production of the aromatic compound phenol. This is done via a process known as oxidative decarboxylation. It can be noted that the ideal temperature under which this process can be carried out is in the range of 300 to 400 °C. Also, benzoic acid and its salts are widely used in the food industry as food preservatives.
Comment on the solubility of benzoic acid in water
Benzoic acid is not very soluble in water. However, the solubility of this compound in water increases when the temperature is increased (as is the case with most compounds). At a temperature of 0°C, the solubility of benzoic acid in water corresponds to 1.7 grams per litre. When heated to 100 °C, the solubility of this compound in water increases to 56.31 grams per litre.
How can benzoic acid be prepared?
Industrially, benzoic acid can be prepared by employing oxygen gas for the partial oxidation of toluene. It can be noted that this process usually employs manganese or cobalt naphthenate as catalysts. This compound can also be prepared via the hydrolysis of benzamide and benzonitrile. It can also be prepared by oxidizing benzyl chloride or benzyl alcohol, or any other derivative of the benzyl group.
Which acid is stronger benzoic acid or acrylic acid?
Ans: The double bond of a benzene ring is less electron donating due to delocalisation which destroys the aromatic character of the benzene ring. Thus, benzoic acid is a stronger acid than acrylic acid.
Why are all aminoacids weaker acids than benzoic acid ?
Ans: Amino group shows +R effect and a weak I effect. Since, -NH₂ group is basic while -COOH group is acidic, therefore, o-aminobenzoic acid undergoes zwitterion formation formation via H bonding. As a result, ortho-effect is reduced to such an extent that o-aminobenzoic acid becomes a weaker acid than benzoic acid and even weaker acid than m-aminobenzoic acid. In fact, due to strong +R -effect, all aminoacids are weaker acids than benzoic acid.
To learn more about this compound and other aromatic compounds, such as pyridine, register with BYJU’S and download the mobile application on your smartphone.


Comments