What is Pyridine?
Pyridine is a heterocyclic compound which is a colourless to yellow liquid with a chemical formula C5H5N.
It is a basic heterocyclic organic compound. It is also known as Azine or Pyridine. The structure is like benzene, with one methine group replaced by a nitrogen atom. It has a sour, putrid, and fish-like odour. Pyridine can be synthesized from ammonia, formaldehyde, and acetaldehyde or it can be made from crude coal tar.
It is weakly basic and is miscible with water. It is highly flammable and when inhaled or ingested it becomes toxic. Some symptoms, when exposed to pyridine, are nausea, asthmatic breathing, vomiting, headache, laryngitis, and coughing. It is widely used in the precursor to agrochemicals and pharmaceuticals. Also, it is used as an important reagent and organic solvent.
Table of Contents
Properties of Pyridine – C5H5N
Pyridine and its simple derivatives are stable and relatively unreactive liquids, with strong penetrating odours that are unpleasant. Pyridine is the hydrogen derivative of this ring, it is benzene in which one CH- or methine group is replaced by a nitrogen atom. The structure of pyridine is completely analogous to that of benzene, being related by the replacement of CH with N.
| C5H5N | Pyridine |
| Molecular Weight/ Molar Mass | 79.1 g/mol |
| Density | 982 kg/m³ |
| Boiling Point | 115 °C |
| Melting Point | −41.6 °C |
Pyridine Structure – C5H5N
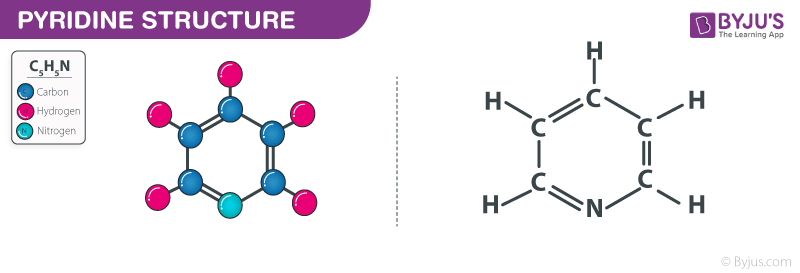
Structure of Pyridine – C5H5N
C5H5N Uses (Pyridine)
- It is used in the chemical industries as a very important raw material
- It is used as an antiseptic in dental care products
- It is used as a solvent which is suitable for dehalogenation
- It is used in pharmaceuticals
- It is used for antifreeze mixtures as a denaturant
- It is used as a sulfonating agent
- It is used as a reducing agent
- It is used in dyes and paints
- It is used as a disinfectant
- It is used in coordination chemistry as a ligand
FAQs
1. Is pyridine an aromatic compound?
Yes, pyridine is an aromatic compound.
2. Is pyridine a good Nucleophile?
No, pyridine is not a good nucleophile. The lone pair on nitrogen is delocalized to some extent around the ring. Then the pyridine is not a strong base. Hence pyridine is a poor nucleophile.
3. Pyridine is more basic than pyrrole. Why?
Pyridine is more fundamental than pyrrole since in pyridine and pyrrole lone pairs of electrons on N are different in nature. Hence, an H+ ion or a Lewis acid can be conveniently transferred to the lone pair of electrons on the N atom in pyridine. Pyridine is also a better base than pyrrole.
4. How is pyridine made?
Pyridine is a chemical product composed of acetaldehyde, ammonia and formaldehyde, combined with a catalyst and reacted at atmospheric pressure of 250-500 degrees Celsius. It is used in pharmaceuticals, solvents and dyes.
5. How many resonating structures does pyridine have?
Pyridine has five resonance structures. The five resonance structures which contain positively charged carbons show this fact. As a result, electrophilic substitution reaction rates at pyridine are significantly lower than those at benzene for electrophilic substitutes.
Also, Read:
| Urea | Carbohydrates |
| Glucose | Phenol |
Learn more about the different applications, structure, and properties of Pyridine (C5H5N) from the expert faculties at BYJU’S – India’s largest education company.

Comments